2017 Volume 3 Issue 3 Pages 48-54
2017 Volume 3 Issue 3 Pages 48-54
Objectives: Andrographis paniculata (A. paniculata) is a widely used herb that has potential medical properties. Andrographolide (Andro) is the major component of A. paniculata. We evaluated the anti-tumor activity of Andro using leukemic cell line cells.
Methods: Leukemic cell lines U937, HL60 or H929 cells were cultured in the presence or absence of Andro and compared with the effects of Ara-C or vincristine. The anti-tumor activity was assessed by morphological observations of the cells, DNA fragmentation, MTT assay, Annexin V positive rate, caspase-3/7 activity, and cell cycle analysis.
Results: After addition of Andro, the morphology of cells changed to characteristic shapes with apoptotic bodies. Furthermore, the Annexin V positive rate and caspase-3/7 activities were increased compared with untreated cells. The G1 phase of cell cycle was also similarly increased compared with cells treated with Ara-C.
Conclusions: Our results show that Andro has an anti-tumor activity against leukemic cell lines, very possibly by inducing apoptosis.
Medicinal plants and plant-derived drugs are potential sources of alternative medicine used to treat various health conditions.1 Plant-derived natural products have served as important drugs in the field of cancer chemotherapy. The plant-derived anticancer drugs vincristine (VCR) and vinblastine have brought invaluable contributions in modern medicine, and function by inhibiting the separation of cell chromosomes.2–4
Andrographis paniculata (A. paniculata), a plant found in China, India and Thailand, is a famous and widely used medicinal herb plant worldwide. Andrographolide (Andro) is the major component of A. paniculata and is mainly concentrated in leaves.1 Andro has been reported to exhibit therapeutic effects in a variety of diseases.5 This plant is considered as an important source of phytomedicine to treat a wide range of diseases, such as respiratory infection, fever, bacterial dysentery and diarrhea.5
Previous studies showed that Andro inhibits proliferation and induces apoptosis in leukemia cell line cells.6 Furthermore, Andro has been shown to exhibit anti-inflammatory activity, through inhibition of lipopolysaccharide (LPS)-induced TNF-α and interleukin-6 (IL-6).7 Recently, the extract of A. paniculata is being used as a nutritional supplement to prevent inflammatory disease in Taiwan.8 In solid tumors, many studies have reported anti-tumor activities of Andro,9–12 but few have examined its effects in leukemic cells. Hence, in this study, we evaluated the anti-tumor activities of Andro on human leukemic cell line cells. Cytosine β-D-arabinofuranoside (Ara-C) is an anti-cancer chemotherapy drug for leukemia, including acute myelogenous leukemia. We focused on the anti-tumor mechanism of Andro compared with cytosine β-D-arabinofuranoside (Ara-C) or VCR as a potential chemotherapy drug for leukemia.
Andrographolide (Tokyo Chemical Industry, Tokyo, Japan) was dissolved at 10 mM in ethanol and used at 10–50 μM. Ara-C from SIGMA-ALDRICH (MO, USA) was dissolved at 10 mg/mL in phosphate-buffered saline (PBS; 150 mM NaCl, 10 mM phosphate-buffer, pH 7.2) and used at 1 μg/mL. Ara-C inhibits the synthesis of DNA. VCR was obtained from SIGMA-ALDRICH, dissolved at 1 mg/mL in PBS, and used at 0.1 μg/mL. VCR binds to tubulin and inhibits microtubule assembly. Ara-C or VCR treatment was used at the concentration to induce about 50% apoptosis with reference to previous experiments.13,14
Cell cultureU937 cells (human monocytic leukemia cell line; EC85011440), HL60 cells (human promyelocytic leukemia cell line; EC98070106) and NCI-H929 cells (human IgA-producing plasma cell line; EC95050415) were obtained from DS PHARMA BIOMEDICAL (Osaka, Japan) and grown in RPMI 1640 medium (Sigma-Aldrich) supplemented with 10% fetal bovine serum (FBS; Equitech-Bio Inc., Kerrville, USA), 100 U/mL of penicillin, and 100 μg/mL of streptomycin (GIBCO, Carlsbad, USA) at 37°C with 5% CO2.
Morphological observation and DNA fragmentationCells were cultured in the presence or absence of Andro, Ara-C or VCR for 24 h. Cells were then deposited on glass slides by the cytospin method at 40×g for 5 min. The glass slides were fixed with Wright solution and stained with Giemsa solution for observing morphological changes.
For the DNA fragmentation assay, cells (1×106 cells) cultured in the presence or absence of Andro, Ara-C or VCR were collected by centrifugation at 300×g for 5 min and washed once with PBS. The cell pellet was suspended in 100 μL of cell lysis buffer (10 mM Tris-HCl buffer, pH 7.4 containing 10 mM EDTA and 0.5% Triton X-100), kept at 4°C for 10 min, and the cell lysate was centrifuged at 16,000×g rpm for 20 min. The supernatants were incubated with RNase A (0.4 mg/mL; Sigma-Aldrich) at 37°C for 60 min, and then with proteinase K (0.4 mg/mL; Sigma-Aldrich) at 37°C for 60 min. Samples were then incubated with 20 μL of 5 M NaCl and 120 μL of isopropyl alcohol at –30°C overnight. Precipitate was then collected by centrifugation at 16,000×g for 15 min and washed by 70% ethanol twice. The samples were allowed to stand for 5 min in a clean bench to dry, and DNA samples thus obtained were dissolved in TE buffer (10 mM Tris-HCl, pH 7.4 and 1 mM EDTA, pH 8.0), and subjected to 2% agarose gel electrophoresis at 100 V for 45 min. DNA was stained with 0.1 mg/L ethidium bromide solution.
MTT assayMTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay was performed using a MTT cell proliferation assay kit (Cayman Chemical Company, Ann Arbor, USA). Cells incubated in the presence or absence of Andro, Ara-C or VCR were seeded at a density of 2×104 cells/well in 100 μL of culture medium in a 96-well plate (Becton and Dickinson) and incubated at 37°C for 24 h. Next, 10 μL of MTT reagent was added to each well, and after mixing gently, the cells were incubated at 37°C for 4 h in a CO2 incubator. After removing 85 μL of supernatant, 100 μL of lysis buffer was added and mixed in the cell solution. Optical density was measured (550 nm) using a microplate reader (BIO-RAD, Benchmark, Hercules, USA).
Quantification of apoptosis by Annexin VDetection of apoptosis was performed using The MuseTM Annexin V and Dead Cell Assay Kit (Merck Millipore Corporation, Darmstadt, Germany) and Muse Cell Analyzer (Merck Millipore Corporation), according to the manufacturer’s protocols. Cells incubated in the presence or absence of Andro, Ara-C or VCR for 24 h were collected by centrifugation (300×g at 4°C for 5 min), suspended in 100 μL of RPMI 1640 medium, and incubated with 100 μL of Annexin V reagent at room temperature for 20 min. Cells were measured by the Muse Cell Analyzer.
Measurement of caspase-3/7 activitiesCells incubated in the presence or absence of Andro, Ara-C or VCR were seeded for 24 h at a concentration of 2×105 cells/mL in a 24-well plate dish (Falcon). Cells were collected by centrifugation (300×g at 4°C for 5 min), and suspended in 50 μl of RPMI 1640 medium. Cells were incubated with 5 μl of caspase-3/7 working solution for 30 min at room temperature. Finally, caspase 7-amino actinomycin D (7-AAD) working solution (2 μL of 7-AAD stock solution, 148 μ1 of 1×Assay Buffer BA) was added, and measured by the Muse Cell Analyzer.
Cell cycle analysisCells (1×106 cells in 3 mL) incubated in the presence or absence of Andro, Ara-C or VCR for 24 h were collected by centrifugation (300×g at room temperature for 5 min), resuspended in 30 μL of PBS and fixed by 200 μL of 80% ethanol (final 70% ethanol concentration) for over 3 h at –20°C. After fixation, cell pellets obtained by centrifugation (300×g, 5 min) were suspended in 500 μL of PBS, and incubated with 200 μL of Muse Cell Cycle Reagents in the dark for 30 min and measured by the Muse Cell Analyzer.
Statistical analysisData were analyzed using Excel software and the student’s t-test was used to assess statistical significance between treatments. Results are expressed as mean±SD of three independent experiments. P<0.05 was considered as statistically significant.
Chromatin structure and nuclear fragmentation were observed by Wright-Giemsa staining in U937 cells incubated with Ara-C (1.0 μg/mL) or Andro (50 μM) for 24 h. The morphological changes by Andro resembled the changes of DNA fragmentation induced by Ara-C treatment (Figure 1). The appearance of DNA fragmentation was observed by agarose gel electrophoresis in U937, HL60 and H929 cells treated with Ara-C (1.0 μg/mL), VCR (0.1 μg/mL) or Andro (50 μM) (Figure 2).

Morphological changes in U937 cells treated with Andro. Apoptotic bodies with nuclear fragmentation were observed after 24 h of treatment with Ara-C or Andro as compared with untreated cells in Wright-Giemsa staining. (a) Untreated cells. (b) Andro treatment. (c) Ara-C treatment.

Detection of DNA fragmentation in cells treated with Andro. Nuclear DNA fragmentations were detected by agarose gel electrophoresis after 24 h of treatment with Ara-C, VCR or Andro. Lane 1: DNA size marker; lanes 2, 6, 10: untreated; lanes 3, 7, 11: Ara-C (1.0 μg/mL), lanes 4, 8, 12: VCR (0.1 μg/mL); lanes 5, 9, 13: Andro (50 μM).
We performed MTT assay to measure the inhibition rate of cell proliferation and viability of the cells incubated with Ara-C, VCR or Andro for 24 h. As shown in Figure 3, the proliferation of all cells was inhibited by the treatment with Ara-C, VCR or Andro for 24 h. Inhibition rates of Andro (50 μM for 24 h) on cell proliferation in each leukemic cell line were 85.5% in U937 cells, 76.3% in HL60 cells and 86.5% in H929 cells compared with untreated cells. These rates were higher than those of Ara-C (1.0 μg/mL) and VCR (0.1 μg /mL) treatment.

Inhibition of cell proliferation by MTT assay. The histogram indicates the optical density after 24 h of treatment with Ara-C, VCR or Andro. The optical density was similarly decreased by the addition of Andro compared with Ara-C or VCR treatment in each leukemic cell line. The results are expressed as mean±SD of three independent experiments ***P<0.001, as compared with untreated cells.
Proportions of annexin V-positive cells after 24 h of treatment with Ara-C, VCR and Andro are shown in Figure 4. The proportions of Annexin V positive cells in cells incubated with Andro (50 μM) were 55.1% in U937 cells, 76.0% in HL60 cells and 97.8% in H929 cells. This rate was increased in a concentration-dependent manner.
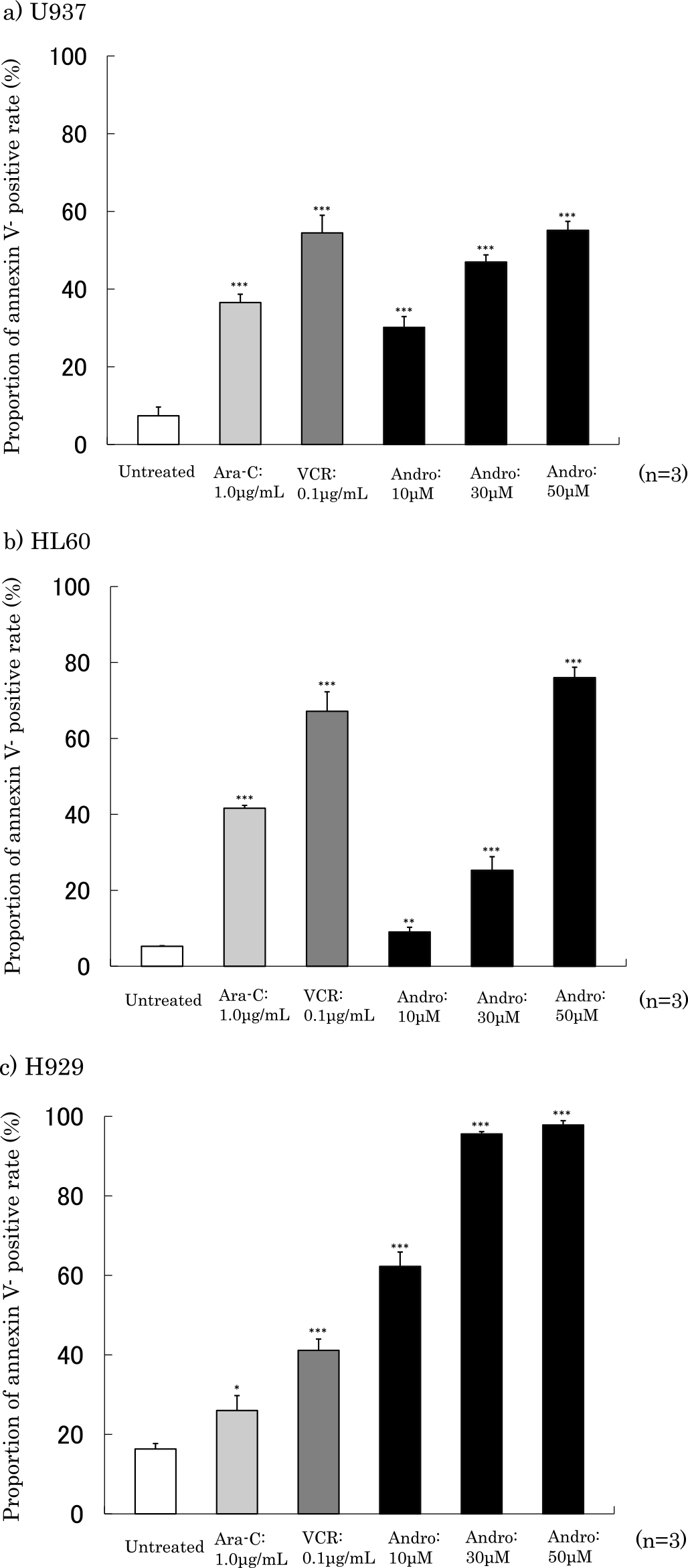
Proportion of annexin V-positive cells. The annexin V-positive proportion was shown after 24 h of treatment with Ara-V, VCR or Andro using the Muse Cell Analyzer. The annexin V-positive proportion indicates the total of annexin V-positive cells with 7-AAD negative and 7-AAD positive cells. The results are expressed as mean±SD of three independent experiments ***P<0.001, **P<0.01, *P<0.05, as compared with untreated cells. (a) U937 cells. (b) HL60 cells. (c) H929 cells.
Dot plots of Annexin V-positive cells showed a clearly increase after addition of Ara-C, VCR or Andro compared with untreated cells (Figure 5).

Dot plots of annexin V-positive cells. Dot plots of annexin V-positive cells are shown after 24 h of treatment with Ara-C, VCR or Andro using the Muse Cell Analyzer. The x-axis indicates the Annexin V-positive cells, and the y-axis indicates 7-AAD positive cells. Each figure shows untreated cells, and Ara-C, VCR or Andro treatment for 24 h. (a) U937 cells. (b) HL60 cells. (c) H929 cells.
We next examined caspase-3/7 activities after 24 h of treatment with Ara-C, VCR or Andro in U937, HL60 or H929 cells (Figure 6). The proportions of caspase-3/7 positive activities after addition of Andro was 51.4% in U937 cells, 51.0% in HL60 cells and 97.3% in H929 cells. We observed a similar increase after addition of Ara-C or VCR compared with untreated cells.
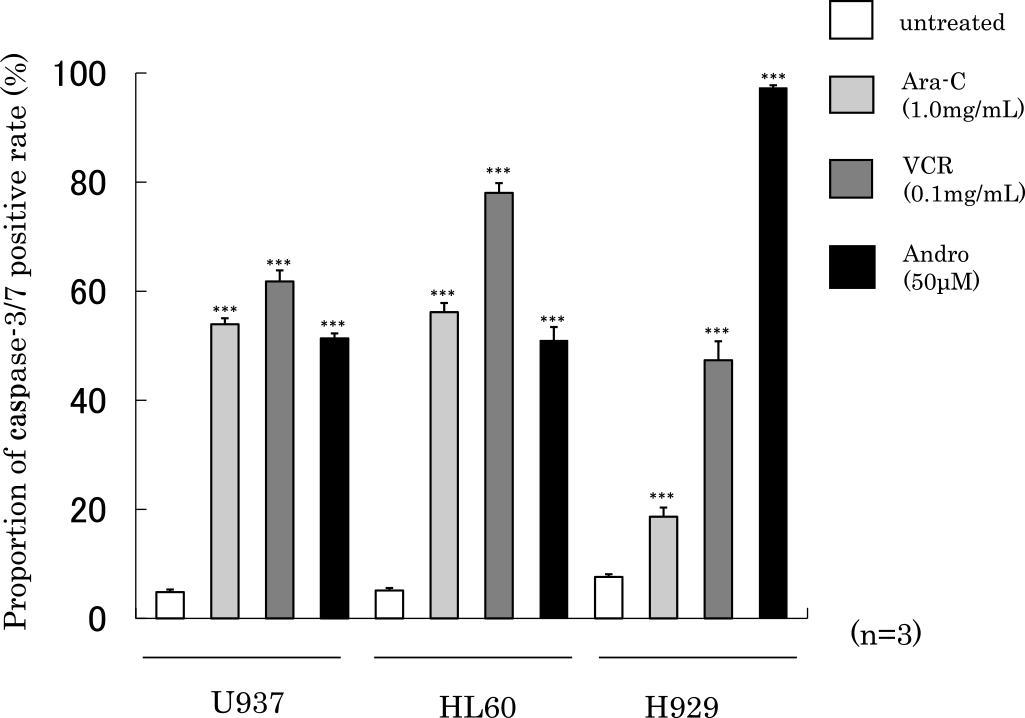
Activation of caspase-3/7. Proportion of caspase-3/7 positive rate after 24 h of treatment with Ara-C, VCR or Andro was increased compared with untreated cells. The results are expressed as mean±SD of three independent experiments. ***P<0.001, **P<0.01, *P<0.05, as compared with untreated cells.
After addition of Andro, the percent of cells in the G1 phase of cell cycle was 50.1% in U937 cells, 42.1% in HL60 cells or 53.8% in H929 cells (Figure 7), indicating an increase in G1 phase cells compared with each untreated cell. As a positive control, we confirmed increase of G1 phase after addition of Ara-C in each leukemic cell line, and increase of G2/M phase after addition of VCR in U937 or H929 cells.

Cell cycle analysis. Cell cycle analysis after 24 h of treatment with Andro in U937, HL60 or H929 cells compared with each untreated cells. As a positive control, Ara-C was shown to increase in G1 phase of cell cycle, and VCR was shown to increase G2/M phase of cell cycle. (a) U937 cells. (b) HL60 cells. (c) H929 cells.
A. paniculata grows widely in many Asian countries and is a potential medical plant. The extracts of A. paniculata have been shown to possess many pharmacological effects such as anti-cancer and anti-inflammatory activities.15 The major bioactive component extracted from A. paniculata is Andro, a diterpenoid lactone with three hydroxyls at C-3, C-19 and C-14.16 In this study, we examined the anti-tumor mechanism of Andro, focusing on apoptotic induction by Andro in leukemic cells.
Apoptosis is characterized by a series of typical morphological features, such as shrinkage of the cell, fragmentation into membrane-bound apoptotic bodies. Addition of Andro was shown to change the morphology to that of apoptotic cells, and we also detected DNA fragmentation in treated leukemic cells.
In MTT assay, the proliferation of leukemic cells was significantly inhibited compared with untreated cells after Andro treatment. Annexin V allows for the quantitative of early or late apoptosis. In early apoptosis, these are externalization of phosphatidyl serine to the cell surface. Annexin V is a calcium-dependent phospholipid-binding protein with a high affinity for phosphatidyl serine. In late apoptosis, cleavage and degradation of specific cellular proteins occurs, as well as fragmentation of nuclear chromatin and loss of membrane integrity. 7-AAD is a dead cell marker, and allows late apoptosis. The Annexin V positive rate was shown to increase significantly as compared with untreated cells after Andro treatment in leukemic cells.
Caspases, a family of cysteine proteases, are crucial mediators of apoptosis. Caspases are present as inactive pro-enzymes that are activated by proteolytic cleavage. Caspases have been subclassified by their mechanism of action and are either initiator caspases (caspase-8 and -9) or executioner caspases (caspase-3, -6, and -7). The activation of caspase 3/7 induces DNA fragmentations. The Caspase-3/7 positive rate was shown to increase significantly as compared with untreated cells after Andro treatment in leukemic cells.17–19
The cell cycle is important for chemotherapy, because many chemotherapeutic drugs work on cells that are actively replicating. Ara-C damages DNA when the cell cycle in the S phase (synthesis of DNA). VCR inhibits microtubule assembly by binding tubulin and producing a transient G2/M phase block. The Muse cell cycle kit includes the nuclear DNA intercalating stain propidium iodide (PI). In the cell cycle, G0/G1 phase contain two copies of each chromosome, and the G2/M phase contain four copies of double chromosome. The G1 phase of cell cycle was increased after addition of Andro as same as Ara-C treatment in leukemic cells.
The present results, including the morphological observation of nuclear fragmentation using Wright-Giemsa and the occurrence of DNA fragmentation observed using agarose electrophoresis, inhibition of cell proliferation by MTT assay, increase in annexin V-positive cells, and increase in caspase 3/7 activities by Andro treatment, suggested the occurrence of apoptosis in U937, HL60 and H929 cells upon treatment with Andro. Ara-C and VCR, basic anti-cancer drugs used for leukemic treatment, were used as a positive control.
Many reports have examined the anti-tumor activity of Andro. Andro has been reported to induce apoptosis with cell cycle arrest, and its mechanism occurred via a mitochondria-dependent pathway in human acute promyelocytic leukemic cell line HL-60 cells.20 Andro was reported to induce apoptosis in the T-cell acute lymphoblastic leukemia cell line Jurkat.21
In this study, we observed the highest annexin V positive rate in H929 leukemic cell. H929 is a human multiple myeloma cell established from myeloma (IgA kappa) at relapse. Multiple myeloma is an incurable plasma cell malignancy. In the last decades, significant changes were achieved in the field of multiple myeloma treatment. A cardinal change came with the introduction of so-called novel agents bortezomib, thalidomide and lenalidomide.22 Bortezomib is a proteasome inhibitor proven to be clinically useful for multiple myeloma and shows anti-tumor activity through inhibition of NF-kB pathway.23 Andro, a novel nuclear factor-kB inhibitor, was reported to inhibit the growth of multiple myeloma cells.24 Andro has shown to inhibit cancer stem cell activity in multiple myeloma and to inhibit multiple myeloma cells.25
Our findings showed that Andro exhibits anti-tumor activity in leukemic cells by inducing apoptosis. Although the present study focuses on in vitro analyses, our results suggest that Andro is an interesting pharmacophore with anti-cancer activity, and hence has the potential for being developed as a cancer therapeutic agent.
The authors declare no competing financial interests.
This work was supported by Institute of Health and Immunology Science.