2018 Volume 4 Issue 3 Pages 55-60
2018 Volume 4 Issue 3 Pages 55-60
Objectives: Andrographis paniculata (A. paniculata) is a major medicinal herb, and andrographolide (Andro) is the main component of A. paniculata. Here we evaluated the anti-proliferative activity and anti-inflammatory effects of Andro on THP-1human monocytic leukemia cell line.
Methods: THP-1 cells were cultured in the presence or absence of Andro, Ara-C or vincristine. The anti-cancer activities of Andro were assessed by cell morphological changes, DNA fragmentation, MTT assay, annexin V positive rate and caspase-3/7 activities. The inflammatory cytokines interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α) were measured after addition of Andro.
Results: MTT assays showed that Andro inhibited cell proliferation of THP-1 cells. Further, annexin V and caspase-3/7 positive rates were increased by Andro compared with untreated cells, indicating induction of apoptosis. IL-6, TNF-α and nuclear factor-kappa B (NF-κB) were increased by LPS, but the addition of Andro blocked the induction of cytokines by LPS.
Conclusions: These results suggest that Andro has anti-proliferative effects and anti-inflammatory activities via induction of apoptosis and the suppression of NF-κB, respectively.
Andrographis paniculata (A. paniculata) is a well-known and commonly used medicinal herb that grows in China, India and Thailand. This plant contains diterpenoids, lactones and flavonoids.1 Andrographolide (Andro) is the major component of A. paniculata and is mainly concentrated in leaves. Since the plant taste is very bitter, A. paniculata is often called the king of bitters.2
Andro has been used to treat a wide range of diseases, such as respiratory infection, asthma and diarrhea.3 Further, a previous study showed that Andro is effective not only for such general physical disorders but also for solid tumors and blood tumors.4 Andro inhibits the proliferation of gastric cancer cells5 and induces apoptosis in pancreatic cancer.6 In human acute myeloid leukemic HL-60 cells, Andro treatment results in an increase in the number of cells in the G1 cell cycle phase along with elevated expression of Bax and decreased expression of Bcl-2.7 Andro induces apoptosis in human T-cell acute lymphoblastic leukemia Jurkat cells by downregulation of the PI3K/AKT pathway.8 Furthermore, Andro induces apoptosis of multiple myeloma cells via caspase-3 or caspase-9 activation.9
Recent studies have shown an anti-inflammatory effect of Andro and demonstrated that this effect involves suppression of TNF-α or IL-6 by the inhibition of NF-κB.10 TNF-α and IL-6 are major inflammatory mediators and are produced in stimulated macrophages by lipopolysaccharide (LPS) localizing at the outer membrane of Gram-negative bacteria.11 The proinflammatory cytokines, including TNF-α and IL-6, are produced via Toll-like receptor 4 (TLR4) in LPS-activated macrophages. TNF-α, one of the cytokines involved in the acute phase reaction, regulates immune cells and inhibits tumorigenesis by inducing apoptosis or inflammation.12 IL-6 is an important mediator of inflammatory response and is related with differentiation and growth in many tumors.13 These inflammatory cytokines are regulated by NF-κB, a member of the proto-oncogene family. NF-κB controls not only the inflammatory response but also biological processes such as cell survival, proliferation, differentiation, and cell homeostasis.14,15
In this study, we examined the potential activities, including anti-proliferative and anti-inflammatory effects in THP-1 monocytic leukemia cell line.
Andro was purchased from Tokyo Chemical Industry (Tokyo, Japan) and dissolved at 10 mM in ethanol. Cytosine β-D-arabinofuranoside (Ara-C) and vincristine sulfate salt (VCR) were from Sigma-Aldrich (MI, USA). Ara-C was dissolved at 10 mg/mL in phosphate-buffered saline (PBS; 150 mM NaCl, 10 mM phosphate-buffer, pH 7.2) and used at 40 μM. VCR was dissolved at 1 mg/mL in PBS and used at 0.1 μM. LPS derived from Salmonella enterica was purchased from Sigma-Aldrich, and was dissolved at 10 μg/mL in PBS. Carbobenzoxy-valyl-alanyl-aspartyl-[O-methyl]-fluoromethylketone (Z-VAD-FMK), a pan-caspase inhibitor, was purchased from Promega (Tokyo, Japan) and used at 20 μM.
Cell cultureTHP-1 human monocytic leukemia cell line (EC88081201) was obtained from DS Pharma Biomedical (Osaka, Japan) and grown in RPMI 1640 medium (Sigma-Aldrich) supplemented with 10% fetal bovine serum (FBS; Equitech-Bio Inc, Kerrville, TX, USA), 100 U/mL of penicillin, and 100 μg/mL of streptomycin (GIBCO, Carlsbad, CA, USA) at 37°C with 5% CO2.
Morphological observationCells were cultured in the presence or absence of Andro, Ara-C or VCR for 24 h. Cells were then deposited on glass slides by the cytospin method at 40×g for 5 min. The samples were fixed with Wright solution and stained with Giemsa solution for observing morphological changes by the Microscope BX50 (OLMPUS).
DNA fragmentationCells (1×106 cells) were cultured in the presence or absence of Andro, Ara-C or VCR for 24 h. Cells were collected by centrifugation at 300×g for 5 min and washed once with PBS. The cell pellets were suspended in 100 μL of cell lysis buffer (10 mM Tris-HCl buffer, pH 7.4 containing 10 mM EDTA and 0.5% Triton X-100) and kept at 4°C for 1 h, and the cell lysate was centrifuged at 16,000×g for 20 min. The supernatants (100 μL) were incubated with RNase A (0.8 mg/mL; Sigma-Aldrich) at 37°C for 60 min, and then with proteinase K (0.8 mg/mL; Sigma-Aldrich) at 37°C for 60 min. After additions of 20 μL of 5 M NaCl and 120 μL of isopropyl alcohol, samples were mixed and then kept at –30°C overnight. Precipitate was collected by centrifugation at 16,000×g for 15 min and washed by 70% ethanol twice. After removal of ethanol, samples were allowed to stand for 5 min in a clean bench to volatilize ethanol. DNA samples were dissolved in TE buffer (10 mM Tris-HCl, pH 7.4 and 1 mM EDTA, pH 8.0) and then subjected to 2% agarose gel electrophoresis at 100 V for 45 min. Gels were stained with 0.1 mg/mL ethidium bromide solution to visualize DNA.
MTT assayMTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay was performed using a MTT cell proliferation assay kit (Cayman Chemical Company, Ann Arbor, MI, USA). Cells were seeded at a density of 3×104 cells/well in 100 μL of culture medium using 96-well plate (Becton and Dickinson) and incubated in the presence or absence of Andro, Ara-C or VCR at 37°C for 24 h. MTT reagent (10 μL) was added to each well and gently mixed, and the cells were incubated at 37°C for 4 h in a CO2 incubator. The supernatant was removed and lysis buffer (100 μL) was added. The optical density (550 nm) was measured using a microplate reader (Bio-Rad Laboratories, Benchmark, Hercules, CA, USA).
Apoptosis assayApoptosis was analyzed using the MuseTM annexin V and Dead Cell Assay Kit (Merck Millipore Corporation, Darmstadt, Germany) according to the manufacturer’s protocols. Cells were seeded at concentration of 2×105 cells/mL in a 24-well dish (Falcon) and incubated in the presence or absence of Andro, Ara-C or VCR. After 24 h, cells were collected by centrifugation (300×g at 4°C for 5 min) and resuspended in 100 μL of RPMI 1640 medium. Cells were then incubated with 100 μL of annexin V reagent at room temperature for 20 min. Cells were analyzed by a Muse Cell Analyzer (Merck Millipore Corporation).
Caspase-3 and caspase-7 assaysThe MuseTM Caspase-3/7 Assay Kit (Merck Millipore Corporation) was used for the detection of caspase-3/7 activities, according to the manufacturer’s protocol. Cells were seeded at concentration of 2×105 cells/mL in a 24-well plate dish (Falcon) and cultured in the presence or absence of Andro, Ara-C or VCR for 24 h. Cells were collected by centrifugation (300×g at 4°C for 5 min) and resuspended in 50 μL of RPMI 1640 medium. Cells were then incubated with 5 μL of caspase-3/7 working solution (1 μL of MuseTM Caspase3/7 Reagent, 7 μL of 1× PBS) for 30 min at room temperature in the dark. The 7-Amino Actinomycin D (7-AAD) working solution (2 μL of 7-AAD stock solution, 148 μL of 1× Assay Buffer BA) was then added, and cells were evaluated by the Muse Cell Analyzer. The inhibition of caspase-3/7 activities were assayed after addition of the pan-caspase inhibitor Z-VAD-FMK (20 μM).
TNF-α and IL-6 ELISA assaysThe Quantikine ELISA kit (R&D Systems, Minneapolis, MN, USA) was used for the detection of TNF-α and IL-6. TNF-α and IL-6 were measured after addition of LPS (10 μg/mL). Cells (4×105 cells/mL) were pretreated with various concentrations of Andro for 1 h and 10 μg/mL of LPS was added to each well and incubated for 24 h. Cells were removed by centrifugation (650×g at room temperature for 10 min), and TNF-α and IL-6 cytokine levels in the supernatant were determined using the kit according to the manufacturer’s instructions. Optical density was measured (550 nm and 450 nm) using a microplate reader (Bio-Rad Laboratories).
NF-κB (p65) DNA-binding assayThe NF-κB (p65) Transcription Factor Assay kit (Cayman, MI, USA) was used to detect NF-κB p65 DNA-binding activity in nuclear extracts. Cells were incubated with various concentrations of Andro (3–50 μM) for 1 h and stimulated with LPS (10 μg/mL). The amount of NF-κB (p65) was measured using a microplate reader (BIO-RAD).
Statistical analysisData were analyzed using Excel software, and Student’s t-test was used to assess statistical significance between treatments. Results are expressed as mean±SD of three independent experiments. A value of P<0.05 was considered as statistically significant.
We first evaluated the morphology of THP-1 cells treated with Andro (50 μM) for 24 h. Shrinkage, nuclear fragmentation and chromatin condensation were observed in cells treated with Andro (50 μM), Ara-C (40 μM) or VCR (0.1 μM) for 24 h (Figure 1). DNA fragmentation was also observed in THP-1 cells treated with Andro (50 μM), Ara-C (40 μM) or VCR (0.1 μM) for 24 h (Figure 2).

Morphological changes of THP-1 cells treated with Andro. Apoptotic bodies with nuclear fragmentation (arrows) were similarly observed after 24 h treated with Andro as compared with Ara-C or VCR in Wright-Giemsa staining.

Detection of DNA fragmentations induced by Andro. Nuclear DNA fragmentations were detected by agarose gel electrophoresis after treatment with Andro, Ara-C or VCR. Lane 1; DNA size marker, Lane 2; untreated, Lane 3; Andro, Lane 4; Ara-C, Lane 5; VCR.
We then performed MTT assay to observe the effects of various concentrations of Andro on the proliferation of THP-1 cells treated for 24 h. The results showed that Andro (10, 30 or 50 μM) inhibited cell proliferation in a concentration-dependent manner (Figure 3). The inhibitory effect of Andro (10 μM or 30 μM) was comparable to those of Ara-C (40 μM) or VCR (0.1 μM).
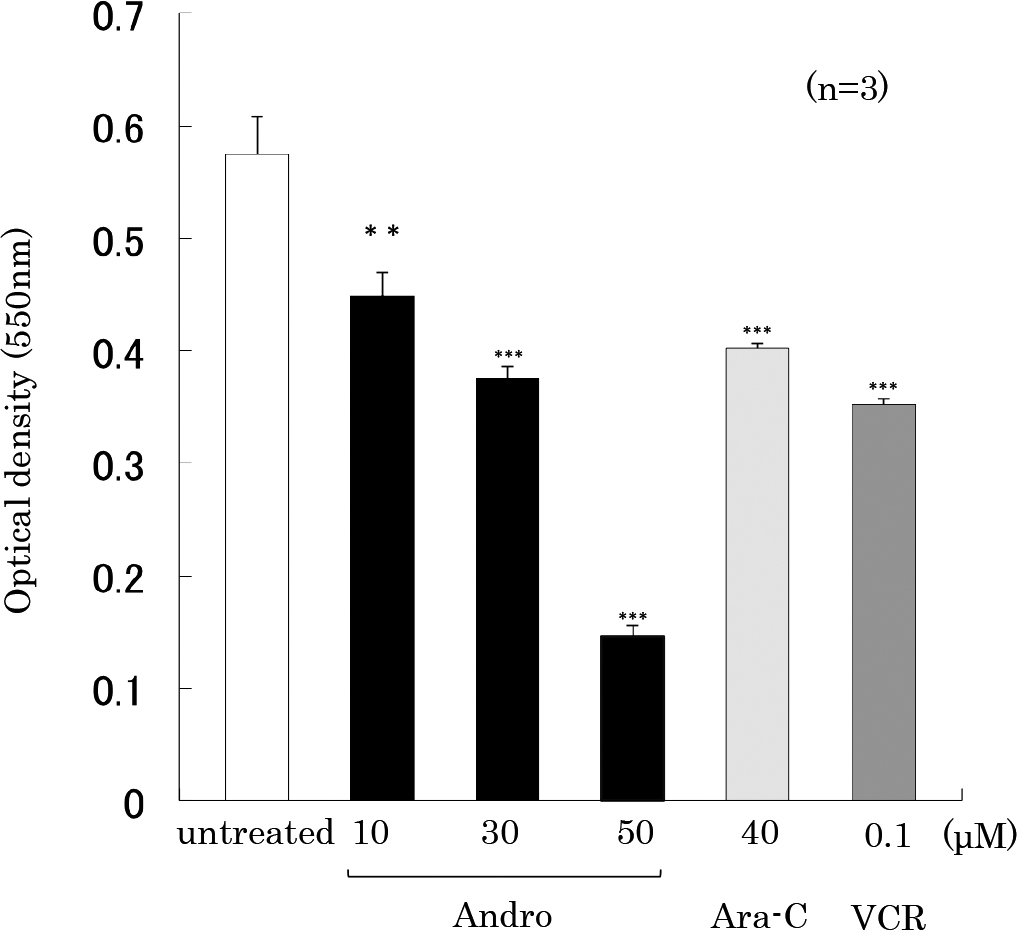
Inhibition of cell proliferation by Andro. The proliferative activities of the cells incubated with Andro, Ara-C and VCR were assessed by MTT assay. The histogram indicates the optical density after 24 h treated with Andro, Ara-C or VCR. Results are expressed as mean±SD of three independent experiments (***P<0.001, **P<0.01, as compared with untreated cells).
Our results showed that Andro effectively induced an inhibition of cell proliferation. To determine whether these effects on cell growth involved apoptosis, we performed annexin V staining and apoptosis analysis (Figure 4). We found that the proportion of annexin V-positive cells after incubation with Andro was increased in a dose-dependent manner (Figure 4a). Andro at 30 and 50 μM showed strong effects compared with Ara-C and VCR treatments.

Effects of Andro on annexinV expression of the cells.
a: Histogram of proportion of annexin V-positive cells. The annexin V-positive proportion was shown after 24 h treatment with Andro, Ara-C or VCR, using the Muse Cell Analyzer. The annexin V-positive proportion indicates the total of annexin V positive cells with 7-AAD negative and 7-AAD positive cells. Results are expressed as mean±SD of three independent experiments (***P<0.001, **P<0.01, as compared with untreated cells).
b: Dot plots of Annexin V positive cells. Annexin V-positive cells after treatment with Andro, Ara-C and VCR for 24 h were shown at dot plots using Muse Cell Analyzer. The x-axis indicates the annexin V-positive cells, and the y-axis indicates 7-AAD positive cells.
The dot plots of annexin V-positive cells are shown in Figure 4b. We found that the percentage of late apoptotic cells (annexin V and 7-AAD double positive area) was increased after treatment with Andro, Ara-C or VCR.
Quantification of caspase-3/7 activation and inhibition by caspase inhibitorWe further examined caspase-3/7 activities in THP-1 cells after treatment with Andro, Ara-C or VCR for 24 h (Figure 5). The proportions of caspase-3/7 positive cells were 85.6% (Andro-treated cells), 38.3% (Ara-C-treated cells), and 60.6% (VCR-treated cells) compared with 9.0% in the untreated control cells. The caspase-3/7 activities induced by Andro, Ara-C or VCR were significantly inhibited by the pan-caspase inhibitor Z-VAD-FMK (Figure 5).

Caspase-3/7 positive rate and inhibition by pan-caspase inhibitor. The caspase-3/7 activities of the cells incubated with Andro, Ara-C or VCR in the presence or absence of Z-VAD-FMK for 24 h were assayed. The caspase-3/7 activities were increased after addition of Andro, Ara-C or VCR, but were significantly inhibited in the presence of pan-caspase inhibitor. Results are expressed as mean±SD of three independent experiments (***P<0.001, as compared with or without inhibitor).
We next measured the inflammatory cytokines of TNF-α and IL-6 in cells stimulated with LPS for 24 h (Figure 6a). TNF-α was increased from 3.1 pg/mL in untreated cells to 234.0 pg/mL with LPS treatment, and IL-6 increased from 0.9 pg/mL to 135.4 pg/mL upon LPS treatment. We then examined whether Andro inhibits the LPS-induced production of TNF-α or IL-6. Cells were pretreated with various concentrations of Andro for 1 h, and LPS was added to each well for 24 h. As shown in Figure 6a, pre-treatment of cells with Andro at 10 μM decreased the LPS-mediated production of both cytokines.

Effects of Andro on the inflammatory cytokine levels stimulated by LPS.
a: Effects of Andro on TNF-α and IL-6 production stimulated by LPS. TNF-α and IL-6 were increased after 24 h treatment of LPS, but Andro inhibited the increase of these inflammatory cytokines in a dose dependent manner.
b: Effects of Andro on NF-κB (p65) activity stimulated by LPS. NF-κB activity was increased by LPS, but this activity was decreased in the presence of Andro. Results are expressed as mean±SD of three independent experiments (***P<0.001, **P<0.01, *P<0.05, as compared with stimulated LPS ).
We further performed ELISA assays to evaluate the effect of Andro on NF-κB activity induced by LPS. NF-κB activity increased after stimulation with LPS, but this induction was reduced by pre-treatment with Andro in a dose-dependent manner (Figure 6b).
In this study, we demonstrated that Andro elicits the anti-proliferative effects in THP-1 monocytic leukemia cells by inducing apoptosis and anti-inflammatory activities by suppressing inflammatory cytokines such as TNF-α and IL-6.
Apoptosis involves activation of the caspase family, resulting in DNA fragmentation and apoptotic body formation.16 We demonstrated that Andro induces morphological changes including nuclear fragmentation and apoptotic body formation in THP-1 cells, similar to effects observed by Ara-C or VCR treatment. We also confirmed that Andro induces DNA fragmentation by agarose gel electrophoresis. In MTT assays, Andro showed an inhibitory effect on cell proliferation. The percentage of cells in the early stage of apoptosis, as detected by annexin V-positivity, increased after Andro treatment. Caspases are a family of cysteine proteases that play an important role in apoptosis stimulated by pro-apoptotic signals. The activation of caspase-3,7 results in the induction of DNA fragmentation. We demonstrated that Andro treatment induced caspase-3/7 activities, and these activities were inhibited by the pan-caspase inhibitor Z-VAD-FMK. These results indicate that the mechanism underlying the anti-proliferative effect of Andro in THP-1 cells occurred through induction of apoptosis through caspase activation. Previous studies reported that Andro causes apoptosis by release of cytochrome c via depolarization of the mitochondrial membrane potential by reactive oxygen species.17 Thus, the mechanism of apoptosis caused by Andro has not been completely clarified, and we will pursue the elucidating of this mechanism in more detail.
The inflammatory cytokines such as TNF-α and IL-6 are important mediators of inflammation that are regulated by NF-κB.18 THP-1 cells are derived from monocytic leukemia and release inflammatory cytokines such as TNF-α and IL-6 via activation of the NF-κB signaling pathway by the stimulation of LPS.19 Our present results suggest that Andro decreases the production of TNF-α and IL-6 stimulated by LPS via the inhibition of NF-κB.
Although the current study presents in vitro results, our findings suggest that Andro is a pharmacophore with anti-proliferative and anti-inflammatory activities that has potential as a cancer and inflammatory therapeutic agent.
The authors declare no competing financial interests.
This work was supported by the Institute of Health and Immunology Science. We thank Gabrielle White Wolf, PhD, from Edanz Group (www.edanzediting.com/ac) for editing a draft of this manuscript.