2018 Volume 4 Issue 3 Pages 70-76
2018 Volume 4 Issue 3 Pages 70-76
Objectives: Reports on the treatment outcome of non-24-hour sleep–wake rhythm disorder (N24SWD) are limited because of low prevalence. We retrospectively analyzed it using stoppage of free-run as the primary index.
Methods: We enrolled 24 consecutive patients who visited the sleep clinic of the Department of Psychiatry of Fujita Health University Hospital and were diagnosed with N24SWD according to the International Classification of Sleep Disorders, Third Edition. Data were retrospectively collected from medical records. When a stopped free-run was identified in an individual during the treatment period, the patient’s clinical state was determined as one of four defined categories based on the extent of the clinical improvement, including “normalization” (i.e., normalized sleep-phase for >3 months). Chronobiological interventions (e.g., bright light therapy, ramelteon administration, and hospitalization) that were considered to have a temporal association with free-run stoppage were also examined.
Results: “Normalization” occurred in 12.5% (3/24 patients) and free-run stoppage occurred in 45.8% (11/24), whereas free-run persisted throughout the course in 45.8% (11/24). The drop-out rate during the treatment course was 54.2% (13/24 patients). No single intervention achieved “normalization,” and patients with free-run stoppage tended to undergo multiple chronobiological interventions.
Conclusions: The very low “normalization” rate and the large number of patients with ongoing free-run who dropped out suggest that N24SWD is extremely refractory. The possibility of free-run stoppage using a combination of multiple chronobiological interventions may be plausible, although we were unable to identify a specific treatment that was effective. Further studies that include the analysis of therapeutic interventions are required.
Circadian rhythm sleep–wake disorder (CRSWD) is a sleep disorder characterized by an inability to synchronize the circadian rhythm of the body with the 24-hour cycle of the external world.1 Many affected patients often have difficulties in both sleep onset and awakening and experience serious problems in their social life, such as difficulties in attending school or work. In non-24-hour sleep–wake rhythm disorder (N24SWD), a very rare type of CRSWD, the sleep–wake rhythm cycle is longer than 24 hours and a progressive 1- to 2-hour delay in the time of sleep onset and awakening occurs every day in typical patients. Accordingly, the sleep–wake pattern becomes counter-phased to environmental day/night and then returns to almost normal. This course is cyclically repeated with a period length of about one month, and is known as “free-run.”2,3 Compared with delayed sleep–wake phase disorder (DSWPD), another representative form of CRSWD, the pathologically delayed circadian sleep–wake cycle associated with the individual’s ability to synchronize is a shared feature; however, the difficulties in social adaptation are more serious in N24SWD because it is more difficult to maintain wakefulness at a consistent time zone because of the free-running sleep–wake rhythm. In the absence of time cues, such as light, the rhythm of the human body has been shown to demonstrate free-running in cycles longer than 24 hours.4 This type of CRSWD is more common among individuals with total blindness,5 but can also be found among sighted persons. However, reports on the clinical features of N24SWD are very limited, and little is known of the specific features of such patients because its prevalence is markedly low. With regard to treatment methodology, there is no established evidence for sighted patients with N24SWD, and there are only case reports of improvement following several treatments including light therapy, melatonin, and melatonin receptor agonists.6,7 Therefore, conventional treatments recommended for DSWPD are often used in combination in the clinical setting, although the symptoms are very refractory. Reports on treatment outcomes in N24SWD patients are even more limited. A previous study of 11 adolescents with CRSWD over a mean follow-up period of 3.7 years included four patients with N24SWD.8 To the best of our knowledge, this study involves the largest sample size, and no other study has investigated the specific course and prognosis in an adequate number of patients with N24SWD. In addition, while bidirectional transition between DSWPD and N24SWD has been reported,9 few studies have documented the frequency of such conversion.
In this study, we retrospectively analyzed the treatment course and outcomes of sighted patients with N24SWD who underwent conventional treatments at the sleep clinic of our department, using stoppage of free-run as the primary index.
This study was conducted as an observational study to retrospectively review existing medical records. We collected data from consecutive patients who first visited the sleep clinic of the Department of Psychiatry of Fujita Health University Hospital (Aichi, Japan) between January 2004 and June 2015, who were diagnosed with N24SWD based on the diagnostic criteria of the International Classification of Sleep Disorders, Third Edition (ICSD-3),9 and who underwent treatment on an outpatient or inpatient basis. The diagnostic criteria for N24SWD in ICSD-3 are shown in Table 1. For the inclusion of patients, we first reviewed the first-visit records of outpatients to extract those with diagnostic terms suggestive of disturbed sleep–wake rhythm. The medical records of the first visits of the extracted patients were then reviewed according to the procedure described in the next section, and the patients were included based on the decision of two psychiatrists. Patients with other concurrent psychiatric or sleep disorders were considered eligible and included as long as CRSWD was the primary target of treatment. Because this was an observational study, each patient received treatment that was within the scope of routine medical care focusing on the adjustment of the internal rhythm (e.g., sleep-hygiene education, light therapy, pharmacotherapy including melatonin receptor agonist or vitamin B12, hospitalization, and other treatments for comorbidities) chosen at the discretion of the treating physician. This study was conducted with the approval of the ethics committee of Fujita Health University. Because this was a retrospective and observational study, the requirement for formal consent from individual subjects was waived.
| Criteria A-D must be met | |
| A) | There is a history of insomnia, excessive daytime sleepiness, or both, which alternate with asymptomatic episodes, due to misalignment between the 24-hour light-dark cycle and the non-entrained endogenous circadian rhythm of sleep-wake propensity. |
| B) | Symptoms persist over the course of at least three months. |
| C) | Daily sleep logs and actigraphy for at least 14 days, preferably longer for blind persons, demonstrate a pattern of sleep and wake times that typically delay each day, with a circadian period that is usually longer than 24 hours. |
| D) | The sleep disturbance is not better explained by another current sleep disorder, medical or neurological disorder, mental disorder, medication use, or substance use disorder. |
Data were retrospectively collected from medical records, including the age at first visit, sex, diagnoses of comorbid psychiatric and/or sleep disorders, and social adaptation before and after treatment. N24SWD was diagnosed based on the diagnostic criteria of ICSD-3, namely, if free-run was considered to have clearly continued for at least 3 months before or after the first visit and to have been confirmed by sleep diary and/or actigraphy for at least 14 days according to medical records. Although ICSD-3 formally requires recordings of both sleep diary and actigraphy for the diagnosis of N24SWD, some earlier patients in this study were diagnosed according to only the sleep diary because we did not necessarily employ actigraphy in diagnosing N24SWD, as in previous versions of ICSD it was not mandatory for the diagnosis of N24SWD.10,11 A delayed sleep phase without free-run before the first visit was classified as a “history of DSWPD” if it persisted for ≥3 months on the description of medical records, or as a “history of suspected DSWPD” if its persistence for ≥3 months was not made clear on the description. The clinical state after the first visit was evaluated as described below.
AnalysisFirst, we determined whether or not free-run stopped for ≥1 month according to the medical records during the entire treatment period of each patient. When a stopped free-run was identified, the duration of stoppage, time of sleep onset, and time of awakening on the medical record were used to categorize the patient into one of four categories according to severity based on the Severity Level Criteria for delayed sleep phase syndrome (DSPS; an alternative name for DSWPD) devised by Ohta et al.12 According to this criterion, 0, 1, 2, or 3 points were allocated when the time of sleep onset was before 0:00, between 0:00 and 2:00, between 2:00 and 4:00, or after 4:00, respectively. Another 0, 1, 2, or 3 points were allocated when the time of awakening was before 8:00, between 8:00 and 9:00, between 9:00 and 10:00, or after 10:00, respectively. The patient was considered to have a severity level of 0 if the cumulative scores for the time of sleep onset and the time of awakening was 0 or 1, a severity level of 1 if the cumulative score was 2 or 3, a severity level of 2 if the cumulative score was 4 or 5, and a severity level of 3 if the cumulative score was 6. When a free-run that stopped for ≥1 month but <3 months, excluding the period of hospitalization, was identified on medical records, the clinical state of the patient was considered to be “semi-normalized” if the severity level was 0 or as “transition to sleep–wake phase delay” if the severity level was 1–3. Moreover, when a free-run that stopped for ≥3 months was identified, the clinical state of the patient was considered as “normalized” if the severity level remained 0; otherwise, the patient was considered as “transition to DSWPD” (Figure 1). A patient was referred to as a “drop-out” if treatment was discontinued upon the patient’s self-judgment or request despite the attending physician’s opinion that treatment should be continued. A patient was referred to as “completed” if the treatment was finished after the attending physician and the patient confirmed the improvement and agreed to end the treatment (including transfer to a different hospital).

Schema of the definitions of clinical states in N24SWD. ⋆Severity criteria for DSPS.10 The yellow boxes are the schematized sleep diary of each clinical state, where the thick black line represents sleep phase and the gray shaded area represents desirable time range for sleep. Bidirectional transition (double-headed arrows) among these clinical states can occur during a course of N24SWD.
According to these criteria, the following overall judgments were made based on the patients’ medical records. (A) Appearance of either clinical state of four categories based on stopped free-run (“normalized,” “semi-normalized,” “transition to DSWPD,” and “transition to sleep–wake phase delay”) over the course of each patient’s treatment; however, if episodes corresponding to multiple categories were found for the same patient, only the episode with the maximum improvement (normalized>semi-normalized>transition to DSWPD>transition to sleep–wake phase delay) was adopted for the patient. (B) For patients in whom free-run stoppage was observed, the association between the clinical state category identified in (A) and any chronobiological intervention (bright light therapy [BL], melatonin administration [MLT], ramelteon administration [RAM], environmental control by hospitalization [HOS], and vitamin B12 administration [VB12]) performed at the time of or immediately before the appearance of the identified clinical state (i.e., free-run stoppage); and for patients in whom free-run stoppage was not observed during the treatment period, any chronobiological intervention technique performed at least once during the treatment period. (C) At the final visit, the above four categories, or “free-running” (continuation of free-run including the transient stoppage for <1 month), or “drop-out”/“completed”; and social adaptation at the first and final visits.
Table 2 provides an outline of the characteristics of the 24 patients enrolled in the study. There were 12 males and 12 females aged 12–44 (mean±SD, 22.2±9.6) years at the time of the initial visit. Thirteen patients presented with a comorbid psychiatric disorder (e.g., mood disorder, developmental disorder; Table 2), and six presented with a comorbid sleep disorder (except CRSWD) (e.g., restless legs syndrome, obstructive sleep apnea syndrome; Table 2). Age at the time of symptom onset was 11–43 (mean, 16.1±7.1) years, and the period from the onset to first visit ranged from 1 month to 23 years (mean, 6.1±6.9 years). The treatment period in our clinic (i.e., observation period) ranged from 1 to 61 months (mean, 1.2±1.4 years). Regarding treatment methods, the chronobiological interventions used include [VB12] for 15 patients, [RAM] for 13, [BL] for 10, [HOS] for 7, and [MLT] for 2 (with overlap), and 2 patients had no specific intervention (except for sleep-hygiene education). Other medications include benzodiazepine/non-benzodiazepine sedative-hypnotics, antipsychotics, midodrine, and antidepressants (with overlap; see Table 2).
| Total | 24 | |
| Gender (M/F) | 12/12 | |
| Age at first visit (yrs) | 22.2±9.6 | |
| Age of onset (yrs) | 16.3±7.1 | |
| Period from onset to initial contact (yrs) | 6.1±6.9 | |
| Follow-up period (yrs) | 1.2±1.4 | |
| Comorbidity (with overlap) | Psychiatric disorders | |
| No comorbidity | 11 | |
| Developmental disorder (susp.) | 4 | |
| Mood disorder, NOS | 3 | |
| Bipolar II disorder | 2 | |
| Major depressive disorder | 1 | |
| Schizophrenia | 1 | |
| Somatoform disorder (susp.) | 1 | |
| Social anxiety disorder | 1 | |
| Panic disorder | 1 | |
| Adjustment disorder | 1 | |
| Sleep disorders | ||
| No comorbidity | 18 | |
| Restless legs syndrome | 3 | |
| Obstructive sleep apnea syndrome (mild) | 2 | |
| Periodic limb movement disorder | 1 | |
| Treatment (with overlap) | Chronobiological interventions | |
| Vitamin B12 [VB12] | 15 | |
| Ramelteon [RAM] | 13 | |
| Bright light therapy [BL] | 10 | |
| Hospitalization [HOS] | 7 | |
| Melatonin [MLT] | 2 | |
| None (education only) | 2 | |
| Other medicines | ||
| Benzodiazepines sedative-hypnotics | 5 | |
| Non-benzodiazepines sedative-hypnotics | 3 | |
| Antipsychotics | 3 | |
| Midodrine | 3 | |
| Antidepressants | 2 | |
| Misc | 4 | |
Values are presented as n or mean±standard deviation.
As described at the initial visit, while 12.5% (3/24 patients) had a “history of DSWPD” before the onset of N24SWD, 37.5% (9/24 patients) had a “history of suspected DSWPD.”
(A) Maximum outcomes during the treatment period (“normalization” or free-run stoppage)During the treatment period, “normalization” occurred at least temporarily in 12.5% (3/24 patients), “semi-normalization” in 8.3% (2/24 patients), “transition to DSWPD” in 25.0% (6/24 patients), and “transition to sleep–wake phase delay” in 0% (0/24 patients), while free-run persisted throughout the course of treatment in 45.8% (11/24 patients; excluding hospitalization period and transient free-run stoppage for <1 month). Among the other two remaining patients, who improved while hospitalized, one was transferred to a different hospital in good condition within 1 month after discharge and the other “dropped out” within 1 month after discharge; therefore, they were not scored as having stoppage of free-run or persistent free-run (Figure 2A). For all three patients who were “normalized,” a period satisfying “transition to DSWPD” was also recorded in a period different from “normalization.”
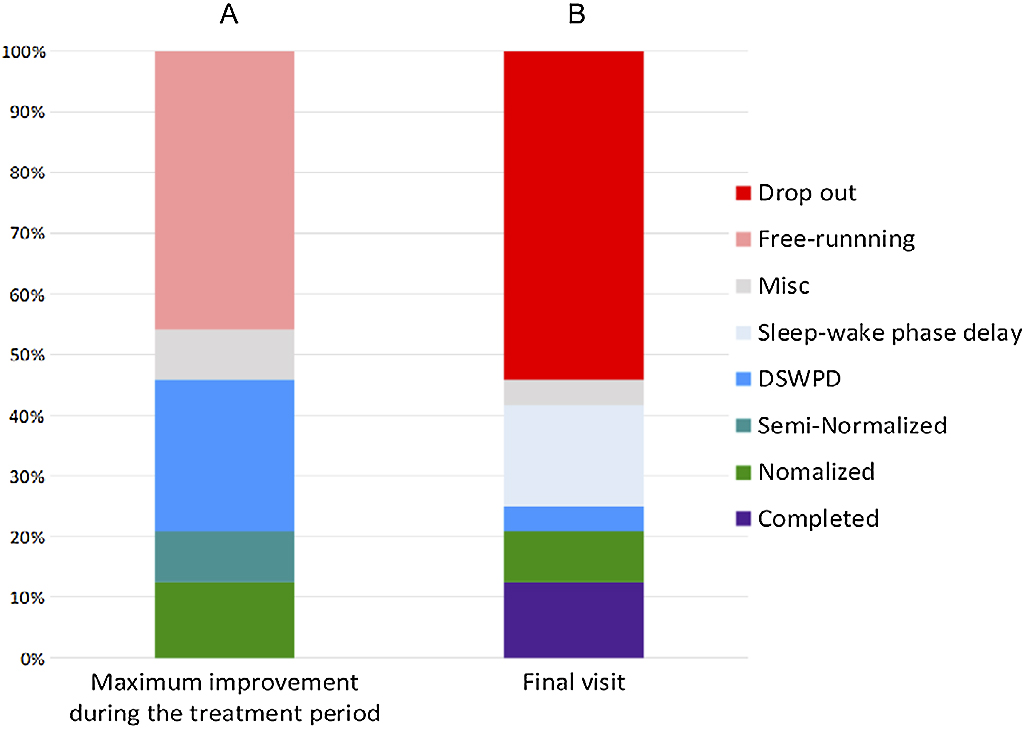
Treatment outcomes. Vertical axis represents the cumulative proportion (%), where 100% is equal to the total number (n=24) of included patients with N24SWD. A. Maximum improvement achieved at any time (excluding the period of hospitalization) during the entire treatment period for each patient. Two patients were classified as “Misc” because they left our clinic within 1 month after improvement by hospitalization. B. Clinical state of each patient at the final visit (i.e., last observation). Patients were not necessarily in their maximally improved clinical states because the states could drift again toward worsening. No patient was in the state of persistent free-running because of dropping out. One patient was classified as “Misc” because the sleep onset/offset times were not clear on the medical record at the final visit.
For three “normalized” patients, the chronobiological interventions performed at the time of or immediately before normalization were [BL+RAM+HOS+VB12] for one patient, [BL+HOS+VB12] for one, and [RAM+VB12] for one. When patients with free-run stoppage (“normalized”+“semi-normalized”+“transition to DSWPD”) were included, treatments were [BL+RAM+HOS+VB12] for two patients, [BL+RAM+HOS] for one, [BL+HOS+VB12] for two, [BL+RAM] for one, [RAM+VB12] for two, [RAM] for two, and [VB12] for one. All multiple interventions were administered simultaneously (Table 3A). In addition to the chronobiological interventions, psychosocial factors seemingly affected free-run stoppage in two patients according to the medical records.
In 11 patients (including “drop-outs”) in whom free-run persisted, the chronobiological interventions performed during the treatment period (including the interventions administered even transiently) were [BL, RAM] for one patient, [RAM, VB12] for two, [MLT, VB12] for one, RAM for one, and VB12 for four, while two patients had no specific intervention (except for sleep-hygiene education). Note that these “tried” interventions were not necessarily administered simultaneously (Table 3B).
Treatment outcomes and chronobiological interventions
| Number of multiple interventions | Chronobiological interventions | Normalized (n=3) | Free-run stoppage (n=11) | ||||
|---|---|---|---|---|---|---|---|
| BL | RAM | MLT | HOS | VB12 | n* | n* | |
| 4 | ● | ● | ● | ● | 1 | 2 | |
| 3 | ● | ● | ● | 1 | |||
| ● | ● | ● | 1 | 2 | |||
| 2 | ● | ● | 1 | ||||
| ● | ● | 1 | 2 | ||||
| 1 | ● | 2 | |||||
| ● | 1 | ||||||
| Number of tried interventions | Chronobiological interventions | Persistent free-running (n=11) | ||||
|---|---|---|---|---|---|---|
| BL | RAM | MLT | HOS | VB12 | n* | |
| 2 | ● | ● | 1 | |||
| ● | ● | 2 | ||||
| ● | ● | 1 | ||||
| 1 | ● | 1 | ||||
| ● | 4 | |||||
| 0 | 2 | |||||
BL, bright light therapy; RAM, ramelteon administration; MLT, melatonin administration; HOS, environmental control by hospitalization; VB12, vitamin B12 administration. Closed circles represent performed interventions: for patients with “normalized” or “free-run stoppage” (“normalized”+“semi-normalized”+“transition to DSWPD”). The multiple interventions shown in Table 3A were performed simultaneously at the time of or immediately before the appearance of the maximum improvement (i.e., stoppage of free-running), but the tried interventions for patients with “persistent free-running” shown in Table 3B were not necessarily administered simultaneously, because they include the interventions performed at least once during the treatment period. The data in the columns “n*” represent the numbers of cases.
At the final visit the proportions of “normalization,” “transition to DSWPD,” and “transition to sleep–wake phase delay” were 8.3% (2/24 patients), 4.2% (1/24), and 16.7% (4/24), respectively. There were 13 “drop-out” patients, three completed the treatment (all of whom were transferred to another hospital), and eight continued to undergo treatment (Figure 2B). Among patients who dropped out, 76.9% (10/13) did so within 6 months.
Regarding social adaptation, all of the patients (24/24) had troubles in school, work, or family life at the first visit. Compared with the first visit, 25.0% (6/24) were considered to have improved in terms of social adaptation at the final visit, and four (50%) were also judged thus among eight patients whose treatment periods were longer than 1 year.
This study reviewed the treatment courses of 24 sighted patients with N24SWD in a mean treatment period of 1.2±1.4 years (ranging from 1 to 61 months) and found that while free-run stoppage occurred at least temporarily in nearly half of them (11 patients), free-run persisted throughout the course in 45.8% (11 patients). During the treatment period, “normalization” for ≥3 months occurred in 12.5% (3/24), and only 25.0% (6/24) presented with improved social adaptation at the final visit. These results suggest the refractoriness of N24SWD. The drop-out rate during the treatment period was 54.2% (13/24), which is also assumed to be related to the refractoriness of N24SWD.
In a previous study conducted by a Nagoya University group, long-term courses of 11 adolescents with CRSWD (seven with DSWPD and four with N24SWD) over a mean period of 3.7 years were investigated, whereby remission occurred in only two patients at the final observation (none with DSWPD and two with N24SWD).8 In the present study, the “normalization” rate at the final observation was 8.3% (2/24), lower than the rate reported in the abovementioned study. However, a direct comparison of these two rates might be inappropriate because our study calculated the “normalization” rate in patients with N24SWD only, including early drop-out patients, whereas the Nagoya University study included a limited number of patients with N24SWD who underwent long-term observation.
In an experiment conducted in an isolated environment, patients with N24SWD reportedly had a prolonged melatonin rhythm compared with healthy individuals with intermediate chronotype.13 In addition, a study in fibroblasts has demonstrated a significantly longer oscillation cycle of the clock gene in patients with N24SWD than in normal healthy individuals and patients with DSWPD.14 These findings suggest that N24SWD may be more refractory than DSWPD because of stronger backward pressure to circadian rhythm than that in DSWPD, resulting in lesser ability to synchronize to an environmental schedule. In addition, patients with N24SWD face challenges in adhering to a schedule for a hospital visit or medication because of progressive backward drift of the sleep–wake phase, which is likely to cause treatment difficulties or high drop-out rates. Approximately three-quarters of patients who dropped out did so within 6 months, suggesting that some patients with N24SWD fall into a vicious cycle such that they drop off because the disorder is refractory and give up on improvement, or the disorder does not improve because they drop off and do not undergo therapeutic intervention.
Bidirectional transition between N24SWD and DSWPDIn a previous study, a group from the National Center of Neurology and Psychiatry in Japan surveyed 57 patients with N24SWD and found that 26% (15 patients) were in the state of DSWPD before the onset of N24SWD.2 In addition, they reviewed relevant previous studies and found that 12.8% of patients (5/39) were in the state of DSWPD before the onset of N24SWD.2 Similarly, Oren et al. reported that two patients underwent a transition from DSWPD to N24SWD during the study course,15 suggesting the possibility of bidirectional transition between these two disorders. In the present study, 50% (12/24) of patients were suspected of having DSWPD before the onset of N24SWD; however, a definitive diagnosis could not be made because prior objective assessments, such as sleep diary or actigraphy, were not performed before patients sought medical help. Conversely, among 24 patients diagnosed with N24SWD, nine (37.5%) had a period without free-run for at least 3 months during the course and were confirmed to have been in the state of DSWPD, including three who achieved “normalization” (the DSWPD status was observed in a period different from when “normalization” occurred). Therefore, results from both this and previous studies suggest that the N24SWD–DSWPD transition can occur frequently.
In the present study, among nine patients who experienced free-run stoppage for ≥3 months and “transition to DSWPD,” a relatively large proportion improved in social functioning in comparison with the first visit (44.4%; four patients) or was confirmed to be normalized (33.3%; three patients). Meanwhile, 11 of 24 patients continued to have free-run and dropped out, among whom as many as 10 dropped out in less than 6 months. These results suggest that while free-run stoppage and “transition to DSWPD” are associated with clinically significant improvement, persistent free-run is associated with drop-out.
Association of “normalization” or free-run stoppage with chronobiological interventionsInsufficient evidence is available for N24SWD regarding therapeutic methodology; treatment methods recommended in the American Academy of Sleep Medicine (AASM) guidelines are extremely limited,6 and conventional treatments for DSWPD are often used in combination. Bright light therapy [BL] is based on the strong circadian rhythm synchronizing effect of light,16 and its use in several cases with N24SWD has been reported,7 including one case successfully treated with [BL] alone17; however, [BL] has “no recommendation” according to the AASM guideline because of insufficient evidence.6 Exogenous melatonin administration [MLT], which has a circadian phase-shifting effect,18 is efficacious for DSWPD.6 There have been several reports of N24SWD cases treated successfully by [MLT],7 and an open trial showed that 5 of 16 patients with N24SWD responded to [MLT].19 However, it is weakly recommended only for blind patients with N24SWD and has “no recommendation” for sighted patients according to the AASM guideline because of insufficient evidence.6 Ramelteon is a melatonin receptor agonist, and its administration [RAM] is expected to exert action similar to that of [MLT], but only two cases of its successful use for N24SWD have been reported, whereby [RAM] was used in combination with other drugs.20 Vitamin B12 administration [VB12], for which success has been reported in N24SWD cases,21,22 has been suggested to regulate the circadian rhythm through endogenous melatonin secretion23,24; however, it too has “no recommendation” according to the AASM guideline.6 Hospitalization [HOS] seemingly improves the circadian rhythm through comprehensive control of environmental factors such as interpersonal contact, light–dark environment, and mealtimes. Although it has been reportedly used successfully in combination with [BL] in Japan,25,26 reports from other countries have been scarce and it is also not described in the AASM guideline. In the present study, [MLT] was used only by two patients who privately imported it because it is not an approved medicine in Japan. In addition, [RAM] was not used before its approval in Japan in 2010, before which [VB12] was frequently utilized.
No patient in our study reported a sufficient improvement via a single intervention. In three patients who achieved the “normalized” state at least once during the course, treatments considered to have a temporal association were [BL+RAM+HOS+VB12], [BL+HOS+VB12], and [RAM+VB12], all of which combine multiple interventions. When free-run stoppage was included (“normalization”+“semi-normalization”+“transition to DSWPD”), the combination of four interventions considered to have temporal association was used for two of 11 patients, a combination of three was used for three patients, a combination of two was used for three patients, and a single intervention was used for three patients. Among the 11 patients who remained in the free-run state, four received two interventions, five received a single intervention, and two dropped out before undergoing chronobiological intervention. These results suggest that these chronobiological interventions can potentially stop the free-run, although it is difficult to attribute this to a specific intervention method based on the present results. Patients who retained free-run over the entire period tended to undergo fewer intervention types; however, the causal relationship (whether they did not exhibit a significant improvement because multiple interventions were not combined, or they did not have an opportunity to receive multiple interventions in combination because they dropped out before any significant improvement could occur) is unclear. Notably, no patients in whom free-run persisted for the entire period involved [HOS]. Although the AASM guideline has “no recommendation” for combinations of multiple interventions,6 given the marked refractoriness of this disorder the combined use of interventions based on different modalities seems rational and may be worth studying further. In addition, two patients in this study displayed a tendency to improve that was triggered by psychological/social factors, which may warrant studies on such interventions in addition to biological interventions.
Limitations of the studyThis study has some limitations. First, the sample size was small. Owing to the rarity of N24SWD, the largest sample size among previous studies conducted in any country was 57,2 and our study is second to it in terms of population. Although the small sample size prevented us from statistically analyzing factors related to the treatment course, most previous reports on the therapeutic course are case reports, and this study may carry specific strength as it studied the therapeutic course in a substantial number of patients. Second, the diagnosis of N24SWD and the evaluation of its symptoms in this study relied on medical records and did not involve direct reference to objective data such as actigraphic recording. Therefore, the accuracy of them may be biased. Moreover, N24SWD-specific features, such as length of sleep–wake cycle and sleep phase jump,2 could not be evaluated. However, because the sleep diary has been used routinely in our diagnosis and treatment of this disorder, the evaluation of the presence of free-running, or its stoppage and normalization, seems to be reliable even with medical record-based assessment. Third, this was a retrospective observational study and it was thus impossible to study the effects of the chronobiological interventions under controlled conditions. It would be appropriate to perform further controlled interventional studies with a larger number of patients. Considering the paucity of currently available evidence and the rarity of N24SWD, the accumulation of case reports with specific focus on its interventions is also warranted.
ConclusionWe conducted a retrospective observational study on the therapeutic course and outcome in 24 consecutive patients with N24SWD. The very low “normalization” rate of 12.5% during the entire treatment period and the large number of patients who dropped out with ongoing free-run suggest that N24SWD is extremely refractory. Nevertheless, free-run was stopped at least temporarily by some interventions in 45.8% of patients. The transition to and from DSWPD was observed in several patients, both before and after the onset of N24SWD. The patients in whom the transition from N24SWD to DSWPD was observed include those having achieved later normalized sleep–wake rhythm and/or improved social function; this finding suggests that free-run stoppage may have a certain clinical significance. Although this study demonstrated the possibility of free-run stoppage under a combination of multiple chronobiological interventions, it could not identify a specific treatment that was effective. Further studies that include analysis of therapeutic interventions are warranted.
The authors report no conflicts of interest related to this research. Dr. Nakao Iwata has received speaker’s honoraria from Astellas, Dainippon Sumitomo, Eli Lilly, GlaxoSmithKline, Janssen, Yoshitomi, Otsuka, Meiji, Shionogi, Novartis, and Pfizer and has received research grants from Dainippon Sumitomo, GlaxoSmithKline, Tanabe-Mitsubishi, and Otsuka. Dr. Tsuyoshi Kitajima has received speaker’s honoraria from Eizai, Tanabe-Mitsubishi, Otsuka, Takeda, Eli Lilly, MSD, Meiji, Yoshitomi, Fukuda, Dainippon Sumitomo, Novo Nordisk, and Shionogi and has received a research grant from Eizai, MSD, and Takeda.