Abstract
Objectives: Alterations in the vaginal bacterial flora reflect the status of various obstetric conditions and are associated with mechanisms that underlie certain pregnancy-associated complications. These changes are also a predictive biomarker for clinical outcomes of these adverse events.
Methods: We examined the vaginal microbiome in samples from pregnant Japanese women with preterm labor.
Results: The microbiota composition in preterm delivery (PD) samples differed from those of control or threatened preterm delivery (TPD) samples in principal component analysis. An increase in Firmicutes and a decrease in Actinobacteria were significantly associated with PD only (both P<0.01). In the Firmicutes phylum, Lactobacillus tended to be abundant, and the abundance of L. iners and L. crispatus was especially high, whereas the L. gasseri population was low in PD samples. Longitudinal analysis showed that the abundance of L. iners decreased after commencing tocolytic treatment in TPD samples compared with before treatment, but it remained high in PD samples.
Conclusions: The vaginal microbiome may be a useful prognostic indicator of preterm labor and a monitoring tool for tocolytic treatment to prevent preterm birth.
Introduction
Preterm delivery (PD) is the main cause of perinatal mortality and morbidity worldwide.1 Preterm infants often suffer from respiratory distress syndrome, necrotizing enterocolitis, and intracranial hemorrhage, which potentially lead to chronic lung disease and developmental delay later in life.2 A major challenge in preventing PD is identifying women at the greatest risk. Among the known maternal and fetal genetic factors, as well as various environment factors, up to 50% of all PD cases are associated with microbial etiologies.3 The most common pathway to intrauterine infection is ascent from the vagina through the cervix into the uterine cavity. Bacterial vaginosis, which is a condition involving an altered vaginal microbiota, has been consistently identified as a risk factor for PD.4 However, the interplay between the microbiota and the host’s physiology remains poorly understood in term and preterm pregnancies. Therefore, to better understand the mechanisms and prognostic biomarkers associated with PD, determining the dynamics of the vaginal microflora is crucial.
Recent advances in metagenomics analysis via high throughput sequencing using next-generation sequencing has enabled evaluation of bacterial diversity among samples under various clinical conditions. Quantitative and qualitative analyses have been conducted using sequence information of 16S ribosomal RNA gene variable regions amplified from vaginal swab samples. Next-generation sequencing-based microbiome studies have provided culture-independent unbiased information on the vaginal microflora. Although a considerable number of studies have been published in this field, inconsistent conclusions have been reached.5–8 To further investigate the possible involvement of bacterial infection in the etiology of PD, we examined the vaginal microbiome and evaluated PD-specific bacterial profiles in a Japanese population. We performed not only cross-sectional analysis but also longitudinal analysis, using a series of samples from each woman with preterm labor in our study to minimize the effect of inter-individual variance in the microbiome. We further assessed whether the identified PD-specific bacterial profile had clinical utility for prognostic prediction of preterm labor. Additionally, we screened for key taxa as biomarkers of the response to tocolytic treatment in women with preterm labor.
Methods
Subjects
All of the clinical samples were collected at the Department of Obstetrics and Gynecology, Fujita Health University Hospital, Japan. Pregnant Japanese women with preterm labor participated in the study. Preterm labor was diagnosed by the presence of at least two uterine contractions every 10 minutes associated with cervical changes in patients with a gestational age of between 20 and 34 weeks. We classified the births in this cohort as PD (delivered at <37 weeks gestation, n=5) or threatened preterm delivery (TPD) (delivered at term, n=12). Women with an uncomplicated pregnancy who were matched for gestational weeks with those who had PD or TPD were also enrolled as a control group (n=23). The clinical details of these women are shown in Table 1. In women who had PD or TPD, continuous intravenous ritodrine hydrochloride treatment at a dose of 50 μg/min was started and the dose was increased to 75 μg/min according to uterine contractions and the cervical length. The infusion rate was increased to a maximum of 200 μg/min. Informed consent was obtained from each patient and this study was approved by the Ethical Review Board for Clinical Studies at Fujita Health University.
Table1
Clinical parameters of the study groups
|
PD |
TPD |
Control |
P value |
| n=5 |
n=12 |
n=23 |
PD vs TPD |
PD vs controls |
TPD vs controls |
| Maternal age (y) |
33.0±2.3 |
32.0±1.8 |
33.9±0.6 |
0.74 |
0.66 |
0.28 |
| Pre-pregnancy body mass index |
21.1±1.3 |
20.3±0.9 |
21.3±0.7 |
0.66 |
0.87 |
0.37 |
| Parity |
1.8±0.5 |
1.3±0.3 |
0.8±0.2 |
0.41 |
0.13 |
0.17 |
| Cervical length (mm) |
12.8±3.1 |
19.3±2.1 |
39.9±1.5 |
0.13 |
<0.01 |
<0.01 |
| Gestational age at sampling (wk) |
27.5±2.7 |
28.0±1.2 |
27.8±1.3 |
0.87 |
0.92 |
0.91 |
| Gestational age at delivery (wk) |
30.5±2.6 |
37.8±0.3 |
38.5±0.3 |
<0.01 |
<0.01 |
0.33 |
| Birth weight (g) |
1592.0±516.1 |
2911.7±158.8 |
3072.0±72.3 |
<0.01 |
<0.01 |
0.38 |
Data are mean±standard error.
Vaginal swab samples were obtained from subjects with PD or TPD at admission and after commencing tocolytic treatment (>30 gestational weeks). Samples were also obtained from women with a normal uncomplicated pregnancy who were matched for the gestational week of the PD and TPD specimens. Briefly, a double-tipped swab (BD BBL CultureSwab plus) was inserted approximately 5 cm into the vagina, pressed against the vaginal sidewall, rotated for 5 seconds, and then removed. Samples were stored at –80°C until later genomic DNA extraction using a QIAamp DNA Microbiome Kit (Qiagen GmbH, Hilden, Germany) in accordance with the manufacturer’s instructions. Seven samples were obtained from 5 women with PD, 20 from 12 women with TPD, and 45 from 23 women with a normal uncomplicated pregnancy.
Polymerase chain reaction amplification and sequencing of 16S rRNA gene V3–4 regions
Polymerase chain reaction (PCR) reactions to amplify the V3–4 regions of bacterial 16S rRNA were conducted using the following universal primers: forward (5'-TCGTCGGCAGCGTCAGATGTGTATAAGAGACAGTCGTCGGCAGCGTCAGATGTGTATAAGAGACAGCCTACGGGNGGCWGCAG-3') and reverse (5'-GTCTCGTGGGCTCGGAGATGTGTATAAGAGACAGGTCTCGTGGGCTCGGAGATGTGTATAAGAGACAGGACTACHVGGGTATCTAATCC-3').9 The bold letters denote the Illumina overhang adapter. Individual samples were barcoded, pooled to construct the sequencing library, and then sequenced using Illumina Miseq (Illumina, San Diego, CA, USA) to generate pair-ended 2×300 reads. Taxonomies were assigned using GreenGene or Ezbio. For further detailed taxonomies, we used a combination of rdp classifier and SpeciateIT.
Statistical analysis
For a β-diversity measure, the weighted UniFrac distance matrix, which measures the pairwise difference in microbial diversity among samples, was calculated using QIIME. To provide visualization of the sample distribution patterns, principal component analysis (PCA) was applied to transform the UniFrac distance matrices into principal coordinates. For volcano plotting, fold changes were calculated using QIIME and the statistical significance was calculated using logistic regression analysis. Intergroup comparisons were made using the Mann–Whitney U test or one-way analysis of variance. Because of the exploratory nature of this study, a P value <0.05 was considered statistically significant, and P>0.05 and <0.1 was recognized as indicating a certain trend toward significance.
Results
We examined the vaginal microbiome of our study subjects by deep sequencing of PCR amplicons of the V3–4 regions in the bacterial 16S rRNA gene. We first conducted PCA analysis for the weighted UniFrac distance matrices to examine whether the global microbiome profile of each sample could differentiate PD from TPD or controls. The PD samples were clustered, while the TPD and control samples were not in clustered this analysis (Figure 1).

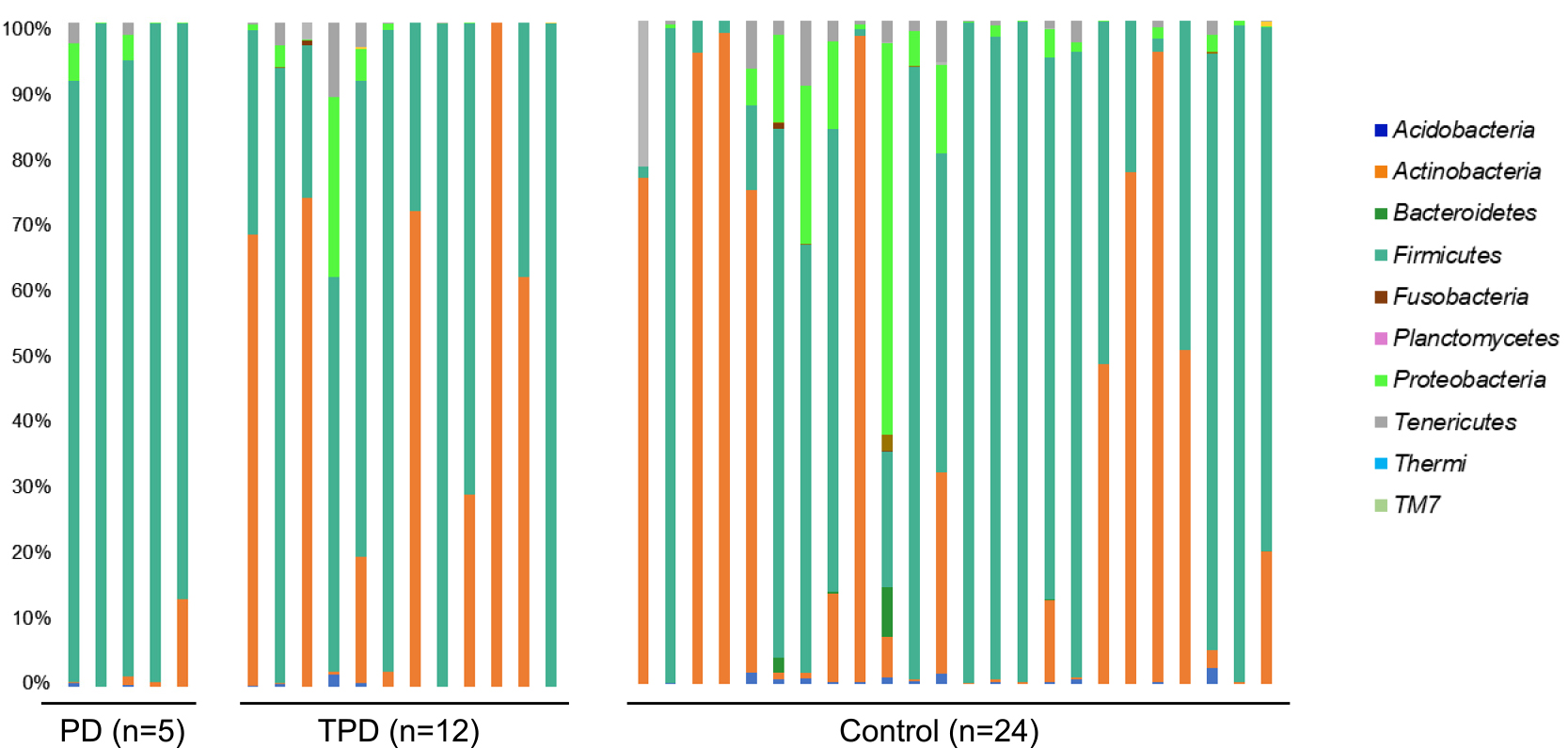
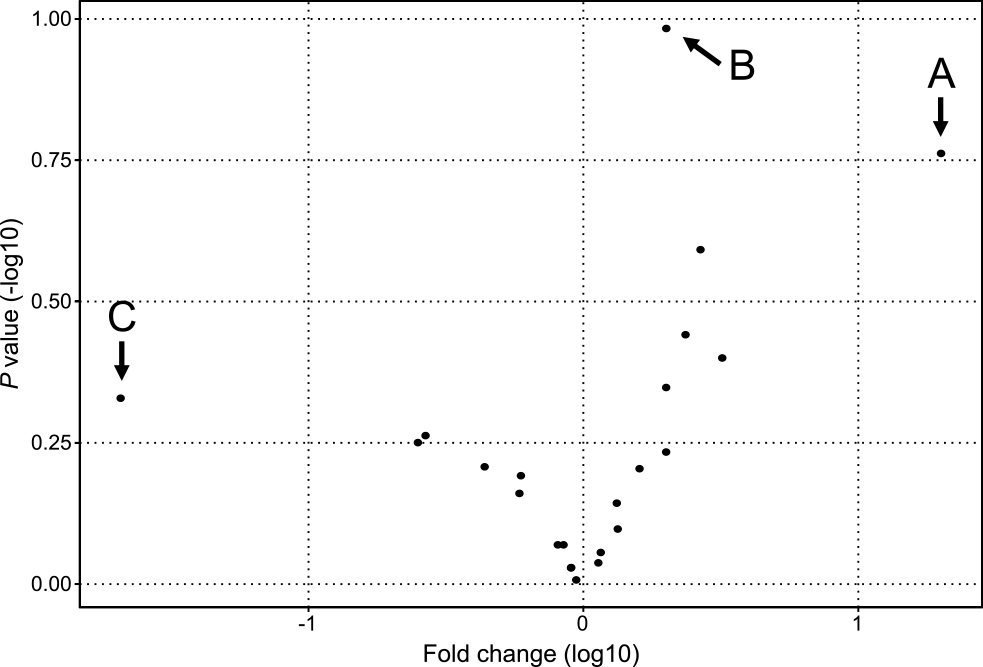
We next compared the relative abundance of the bacterial populations between the PD group and the TPD or control group via taxonomic assignment. Clear differences were observed when we analyzed the data at the bacterial phyla level. Firmicutes was the dominant phylum in the PD group relative to the TPD or control group (P<0.01, PD versus TPD; and P<0.01, PD versus controls), while Actinobacteria was dominant in the TPD and control groups (P<0.01, PD versus TPD; and P<0.01, PD versus controls) (Figure 2). However, when we performed a similar analysis for more detailed bacterial taxa, the differences became statistically unclear. To identify additional bacterial taxa with different abundances between the PD, TPD, and control groups, we performed a similar statistical analysis and expressed the data in a volcano plot. When we analyzed the data for the bacterial genera, three species showed notable trend differences in terms of their abundance between the PD and non PD groups (Figure 3). The sequence reads for Finegoldia were the most abundant in the PD group (20-fold, P=0.17). Lactobacillus showed a lower level than that for Finegoldia, but this still tended to be higher in the PD group than in the control group (2-fold, P=0.10). Because both of these strains belong to the Firmicutes phylum, we speculated that the increase in Finegoldia and Lactobacillus in PD may be responsible for the increase of Firmicutes. In contrast, Bifidobacterium showed the lowest abundance in the PD group, although this was not significant (0.02-fold, P=0.46). We further speculated that the decreased abundance of Bifidobacterium in PD underlies the decrease of the Actinobacteria phylum to which it belongs.
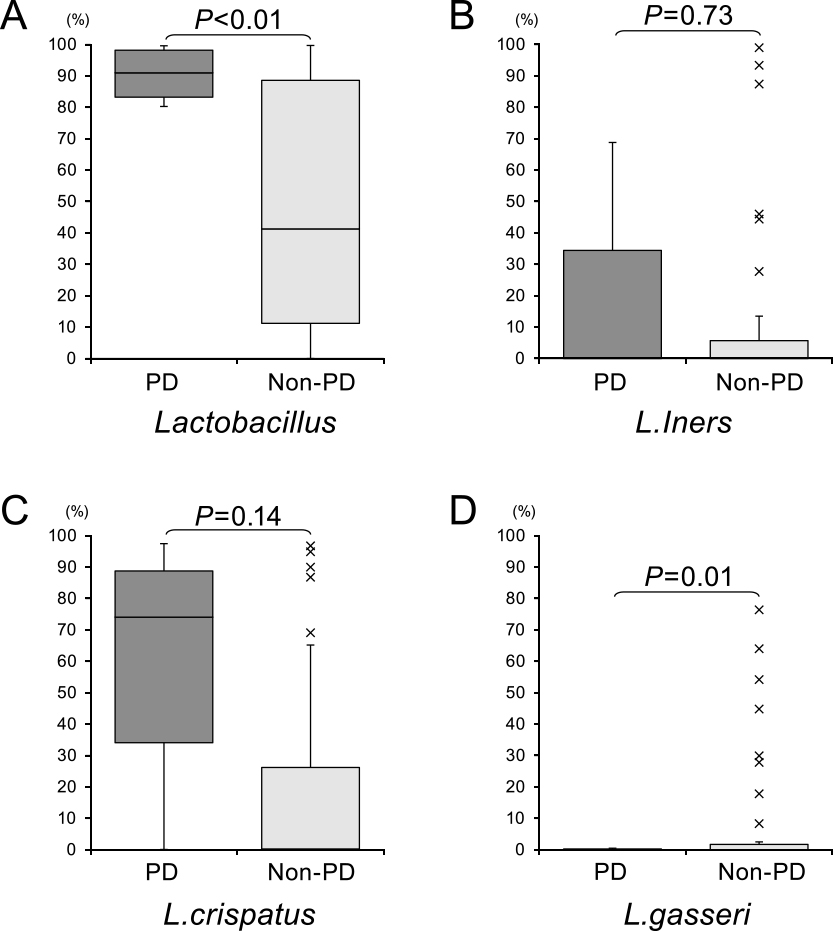
To specify the bacterial species in the vaginal microbiota that were enriched in PD cases, we performed additional sequence analysis with reference to public databases. We then compared the abundance of the identified strains between the PD and control groups. We did not find any specific Finegoldia or Bifidobacterium strains that could have contributed to the high or low abundance of these genera, and thus possibly differentiate PD from either TPD or a normal pregnancy. In our analysis of Lactobacillus species, we found a higher relative abundance of strains of this genus in the PD group among the bacterial taxa (P<0.05). We found that the relative abundance of L. crispatus and L. iners tended to be higher in the PD group, but this was not significant. In contrast, the relative abundance of L. gasseri was significantly lower in the PD group (P<0.05; Figure 4).
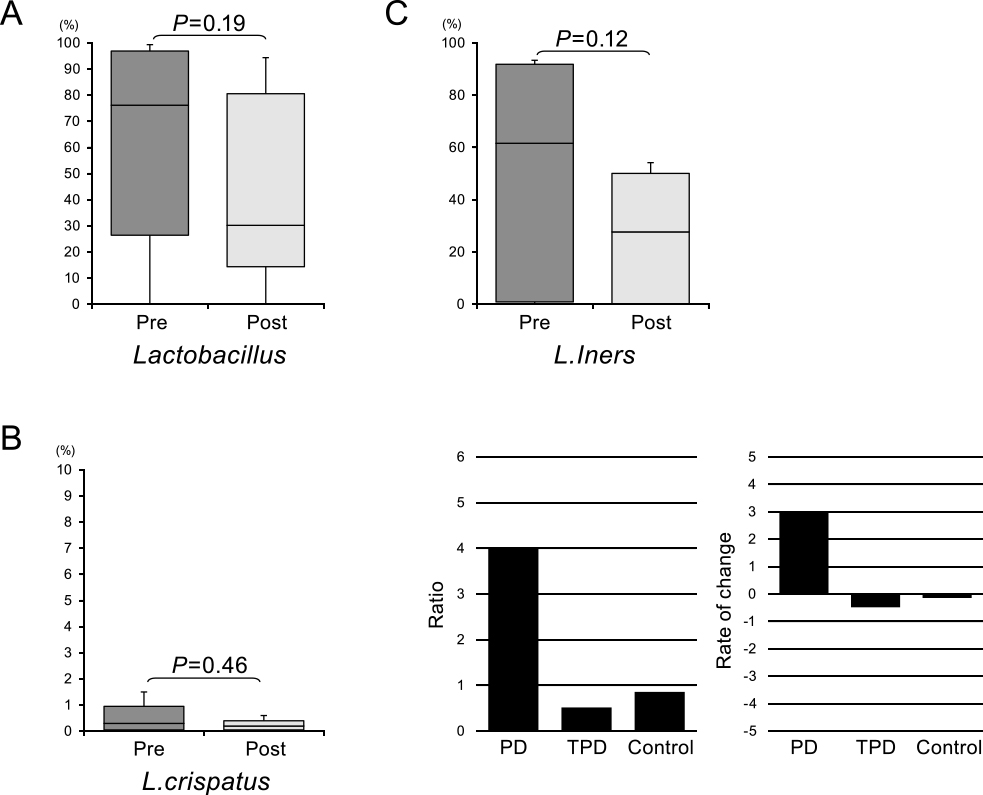
Finally, longitudinal analysis was performed using samples obtained from women with TPD who were included in this study during their course of tocolytic treatment. When we compared the abundance of the various bacterial taxa before and after this therapy, the relative abundance of Lactobacillus genus strains showed a decline after therapy (Figure 5). Moreover, L. crispatus and L. iners species were also lower in this treated group. Notably, while the relative abundance of L. iners was decreased in the TPD group, it was not reduced in the PD group (Figure 5C) or in the control group. Our findings suggest that the reduced abundance of L. iners reflected a response to tocolytic treatment.
Discussion
The maternal vaginal microbiota contributes to the pathophysiology of preterm labor, but conflicting results in recent years have raised doubts about the validity of this relationship. The microbiome is affected by various factors, such as ethnicity, social and demographic parameters, and the vaginal subsites used for sampling.10,11 In our study, to minimalize these variations, we enrolled only Japanese subjects and used similar vaginal subsites for sampling. However, because the microbiome profile varies considerably among individuals, we performed not only cross-sectional analysis, but also longitudinal analysis, which allowed us to better interpret the vaginal microbiome data.
Although PCA showed differences between PD and TPD or control uncomplicated pregnancies, we only found phyla that were specific to PD in our analyses, but not more detailed results for variation in bacterial taxa using standard comparative statistics. Therefore, we used a volcano plot to identify strains of the Lactobacillus genus that differed between PD and non PD samples. Lactobacillus is the most common type of bacterium in the healthy adult vagina, and its abundance at this site increases during pregnancy and does not change or slightly increases with the progression of gestation.5,6,12 Additionally, the abundance level of Lactobacillus declines in PD.6–8,13 However, this study showed that the vaginal richness of Lactobacillus was increased in PD samples. The abundance of Lactobacillus in the vagina has been attributed to the requirement for a lower pH caused by these lactic acid-producing bacteria to protect against pathogenic infection. Although we did not measure the vaginal pH in our cohort, the diversity of Lactobacillus species may be more important that the overall abundance of the Lactobacillus population.14 Differences in the prominence of Lactobacillus strains in the vaginal microbiome may also be simply due to ethnic differences.15
We hypothesize that the balance of different Lactobacillus species, but not their overall abundance, is protective of the vaginal environment, and that an imbalance can lead to PD. Our study indicated that the L. crispatus and L. iners bacterial strains tended to be increased, while the abundance of L. gasseri was decreased in PD. These bacterial decreases in PD have also been well documented previously.16,17 Additionally, L. crispatus contributes to the inhibition of E. coli growth, which suggests that this bacterial strain also plays a protective role against PD.18 Notably, we found that L. iners tended to be increased along with an elevated abundance of L. crispatus in PD. Previous studies have shown that L. iners is associated with a higher risk of PD.8,19 When there is an abundance of L. iners because of its low production of lactic acid, the vaginal microflora do not maintain a low enough pH to be protective. This condition allows a higher diversity of pathological species, which leads to a higher risk of PD onset.20,21 However, some researchers have reported that the richness of L. iners is associated with vaginal cleanliness in terms of pathogens.22 Our study showed that the relative abundance of L. gasseri was significantly decreased in PD, which is consistent with previous findings of a clear negative association between L. iners and L. gasseri.23 However, one study reported that the abundance of L. iners and L. gasseri were decreased in PD.24 Therefore, the clinical significance of the association of these genera with PD in the vaginal microbiome remains unclear.
Finally, we performed longitudinal analysis of the vaginal microbiome in PD and TPD cases and compared pre- and post-treatment samples. When we calculated the post- to pre-treatment ratios, L. iners showed a dynamic change in its abundance. The abundance of L. iners is susceptible to change according to the condition of pregnancy.25 Therefore, we focused on L. iners as a candidate biomarker of PD. One of our investigative approaches was to identify any differences in the abundance of L. iners between pre-treatment PD and TPD samples because we speculated that it might serve as a prognostic marker for tocolytic treatment. The pre-treatment abundance of L. iners in the PD group was higher than that in the TPD group, but this was not significant. Our second approach was to assess any changes in the abundance of L. iners during treatment. The abundance of this bacterium decreased in the TPD group, but not in the PD group, during tocolytic therapy. This finding indicated that the abundance of L. iners is potentially a good biomarker of the response to tocolysis.
An important limitation to this study is the variation in the microbiome profile among individuals, which could be overcome by an increased number of samples. Further and more detailed evaluations of the vaginal microbiome using a greater number of sampling sites from each patient and a larger cohort will help to validate its potential usefulness as a biomarker of PD/TPD. Adding living Lactobacillus species to correct the vaginal bacterial flora might confer a prophylactic benefit against the risk of PD. However, a recent meta-analysis showed no evidence that taking probiotics or prebiotics during pregnancy decreased the risk of preterm delivery.26
In conclusion, the vaginal microbiome may be an effective prognostic indicator of preterm labor and of the effects of tocolytic treatment for preventing preterm birth.
Acknowledgments
We thank Mr. Y. Handa, Bioengineering Lab., Co., Ltd, Kanagawa, Japan for helping NGS analysis. We also thank Mr. T. Ishihara for helping with statistical analysis.
Notes
Conflict of Interests
The authors declare that they have no conflict of interests.
Funding Sources
This study was supported by the Ogyaa Donation Foundation from the Japanese Association of Obstetricians and Gynecologists. This study was also supported by grants-in-aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology, and from the Ministry of Health, Labour and Welfare of Japan.
References
- 1. Blencowe H, Cousens S, Oestergaard MZ, Chou D, Moller AB, Narwal R, Adler A, Vera Garcia C, Rohde S, Say L, Lawn JE. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet 2012; 379: 2162–2172.
- 2. Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet 2008; 371: 75–84.
- 3. Lamont RF, Nhan-Chang CL, Sobel JD, Workowski K, Conde-Agudelo A, Romero R. Treatment of abnormal vaginal flora in early pregnancy with clindamycin for the prevention of spontaneous preterm birth: a systematic review and metaanalysis. Am J Obstet Gynecol 2011; 205: 177–190.
- 4. Leitich H, Bodner-Adler B, Brunbauer M, Kaider A, Egarter C, Husslein P. Bacterial vaginosis as a risk factor for preterm delivery: a meta-analysis. Am J Obstet Gynecol 2003; 189: 139–147.
- 5. Romero R, Hassan SS, Gajer P, Tarca AL, Fadrosh DW, Bieda J, Chaemsaithong P, Miranda J, Chaiworapongsa T, Ravel J. The vaginal microbiota of pregnant women who subsequently have spontaneous preterm labor and delivery and those with a normal delivery at term. Microbiome 2014; 2: 18.
- 6. Hyman RW, Fukushima M, Jiang H, Fung E, Rand L, Johnson B, Vo KC, Caughey AB, Hilton JF, Davis RW, Giudice LC. Diversity of the vaginal microbiome correlates with preterm birth. Reprod Sci 2014; 21: 32–40.
- 7. DiGiulio DB, Callahan BJ, McMurdie PJ, Costello EK, Lyell DJ, Robaczewska A, Sun CL, Goltsman DS, Wong RJ, Shaw G, Stevenson DK, Holmes SP, Relman DA. Temporal and spatial variation of the human microbiota during pregnancy. Proc Natl Acad Sci U S A 2015; 112: 11060–11065.
- 8. Callahan BJ, DiGiulio DB, Goltsman DSA, Sun CL, Costello EK, Jeganathan P, Biggio JR, Wong RJ, Druzin ML, Shaw GM, Stevenson DK, Holmes SP, Relman DA. Replication and refinement of a vaginal microbial signature of preterm birth in two racially distinct cohorts of US women. Proc Natl Acad Sci U S A 2017; 114: 9966–9971.
- 9. Shahi SK, Zarei K, Guseva NV, Mangalam AK. Microbiota Analysis Using Two-step PCR and Next-generation 16S rRNA Gene Sequencing. J Vis Exp 2019; 152: 10.3791/59980.
- 10. Ravel J, Gajer P, Abdo Z, Schneider GM, Koenig SS, McCulle SL, Karlebach S, Gorle R, Russell J, Tacket CO, Brotman RM, Davis CC, Ault K, Peralta L, Forney LJ. Vaginal microbiome of reproductive-age women. Proc Natl Acad Sci U S A 2011; 108: 4680–4687.
- 11. Aagaard K, Petrosino J, Keitel W, Watson M, Katancik J, Garcia N, Patel S, Cutting M, Madden T, Hamilton H, Harris E, Gevers D, Simone G, McInnes P, Versalovic J. The Human Microbiome Project strategy for comprehensive sampling of the human microbiome and why it matters. FASEB J 2013; 27: 1012–1022.
- 12. Aagaard K, Riehle K, Ma J, Segata N, Mistretta TA, Coarfa C, Raza S, Rosenbaum S, Van den Veyver I, Milosavljevic A, Gevers D, Huttenhower C, Petrosino J, Versalovic J. A metagenomic approach to characterization of the vaginal microbiome signature in pregnancy. PLoS One 2012; 7: e36466.
- 13. Blostein F, Gelaye B, Sanchez SE, Williams MA, Foxman B. Vaginal microbiome diversity and preterm birth: results of a nested case-control study in Peru. Ann Epidemiol 2020; 41: 28–34.
- 14. Freitas AC, Bocking A, Hill JE, Money DM; VOGUE Research Group. Increased richness and diversity of the vaginal microbiota and spontaneous preterm birth. Microbiome 2018; 6: 117.
- 15. Serrano MG, Parikh HI, Brooks JP, et al. Racioethnic diversity in the dynamics of the vaginal microbiome during pregnancy. Nat Med 2019; 25: 1001–1011.
- 16. Stafford GP, Parker JL, Amabebe E, Kistler J, Reynolds S, Stern V, Paley M, Anumba DOC. Spontaneous Preterm Birth Is Associated with Differential Expression of Vaginal Metabolites by Lactobacilli-Dominated Microflora. Front Physiol 2017; 8: 615.
- 17. Fettweis JM, Serrano MG, Brooks JP, et al. The vaginal microbiome and preterm birth. Nat Med 2019; 25: 1012–1021.
- 18. Ghartey JP, Smith BC, Chen Z, Buckley N, Lo Y, Ratner AJ, Herold BC, Burk RD. Lactobacillus crispatus dominant vaginal microbiome is associated with inhibitory activity of female genital tract secretions against Escherichia coli. PLoS One 2014; 9: e96659.
- 19. Kindinger LM, Bennett PR, Lee YS, Marchesi JR, Smith A, Cacciatore S, Holmes E, Nicholson JK, Teoh TG, MacIntyre DA. The interaction between vaginal microbiota, cervical length, and vaginal progesterone treatment for preterm birth risk. Microbiome 2017; 5: 6.
- 20. Leizer J, Nasioudis D, Forney LJ, Schneider GM, Gliniewicz K, Boester A, Witkin SS. Properties of Epithelial Cells and Vaginal Secretions in Pregnant Women When Lactobacillus crispatus or Lactobacillus iners Dominate the Vaginal Microbiome. Reprod Sci 2018; 25: 854–860.
- 21. Walsh SW, Nugent WH, Alam SMK, Washington SL, Teves M, Jefferson KK, Strauss JF 3rd. Protease Amplification of the Inflammatory Response Induced by Commensal Bacteria: Implications for Racial Disparity in Term and Preterm Birth. Reprod Sci 2020; 27: 246–259.
- 22. Zheng N, Guo R, Yao Y, Jin M, Cheng Y, Ling Z. Lactobacillus iners Is Associated with Vaginal Dysbiosis in Healthy Pregnant Women: A Preliminary Study. Biomed Res Int 2019; 2019: 6079734.
- 23. De Backer E, Verhelst R, Verstraelen H, Alqumber MA, Burton JP, Tagg JR, Temmerman M, Vaneechoutte M. Quantitative determination by real-time PCR of four vaginal Lactobacillus species, Gardnerella vaginalis and Atopobium vaginae indicates an inverse relationship between L. gasseri and L. iners. BMC Microbiol 2007; 7: 115.
- 24. Tabatabaei N, Eren AM, Barreiro LB, Yotova V, Dumaine A, Allard C, Fraser WD. Vaginal microbiome in early pregnancy and subsequent risk of spontaneous preterm birth: a case-control study. BJOG 2019; 126: 349–358.
- 25. Verstraelen H, Verhelst R, Claeys G, De Backer E, Temmerman M, Vaneechoutte M. Longitudinal analysis of the vaginal microflora in pregnancy suggests that L. crispatus promotes the stability of the normal vaginal microflora and that L. gasseri and/or L. iners are more conducive to the occurrence of abnormal vaginal microflora. BMC Microbiol 2009; 9: 116.
- 26. Jarde A, Lewis-Mikhael AM, Moayyedi P, Stearns JC, Collins SM, Beyene J, McDonald SD. Pregnancy outcomes in women taking probiotics or prebiotics: a systematic review and meta-analysis. BMC Pregnancy Childbirth 2018; 18: 14.