2014 Volume 60 Issue 1 Pages 68-74
2014 Volume 60 Issue 1 Pages 68-74
[Aim] The efficacy of pre-procedure oral proton pump inhibitor (PPI) administration for gastric endoscopic submucosal dissection (ESD) is unclear. This study evaluated oral PPI administration effectiveness on the day of ESD to prevent post-ESD bleeding. [Methods] This study examined 55 patients who underwent ESD for gastric neoplasm. Group A comprised 31 patients who took rabeprazole sodium (RPZ) 20 mg/day beginning 7-8 hr before ESD. Group B comprised 24 who took RPZ 20 mg/day beginning three days before ESD. Gastric pH (G-pH) was measured at one month before ESD (pre-ESD pH), immediately before ESD (ESD pH), and seven days after ESD (post-ESD pH). The post-ESD bleeding rate and changes in G-pH were recorded. [Results] No significant difference in post-ESD bleeding rates was found (Group A 3.2%, Group B 0%). ESD pH and post-ESD pH were significantly higher than pre-ESD pH in both groups (P<0.001). The ESD pH for Group A was higher than 6 (6.5±1.1), providing hemostasis for intragastric bleeding. [Conclusions] Oral RPZ administration on the day of gastric ESD can suppress post-ESD bleeding equivalently to administration three days before ESD.
Endoscopic submucosal dissection (ESD) has become the standard endoscopic resection technique for early gastric cancer in Japan. The most important benefit of ESD is that it enables the removal of large specimens. However, post-ESD bleeding sometimes occurs (5-13%) because a large iatrogenic ulcer can be induced by ESD1-6). To prevent that bleeding, vessels on an iatrogenic ulcer must be coagulated. Furthermore, the intragastric pH (G-pH) must be raised. For hemostasis when intragastric bleeding occurs, the G-pH must be raised above 67). Gastric mucosal bleeding drops markedly at G-pH >6.48). Therefore, when an iatrogenic ulcer occurs after ESD, it is important to maintain G-pH >6 to prevent post-ESD bleeding. Because proton pump inhibitor (PPI) is superior to Histamin-2 receptor antagonist (H2RA) in inhibiting gastric acid, PPI should be administered before ESD. Recent reports have indicated that PPI can be effective for preventing post-ESD bleeding. However, in most studies9-18), the method used was intravenous PPI injection immediately after ESD. We inferred that oral administration of PPI would be easier than intravenous injection, but only three reports have described oral PPI administration for ESD19-21). Watanabe et al.19) reported that oral PPI administration before ESD was useful for preventing post-ESD bleeding, noting that post-ESD bleeding occurred in 0% of patients given lansoprazole (LPZ) orally for a week before ESD compared to 6.4% in those not given PPI. Uedo et al.20) reported that PPI was more effective for preventing post-ESD bleeding than H2RA because post-ESD bleeding occurred in just 1.8% of patients given RPZ orally the day before ESD, although it occurred in 12% of those given cimetidine. Niimi et al.21) reported the effects on ulcer healing of oral RPZ administration starting the day before ESD and continuing for two weeks. Their report described that post-ESD bleeding occurred in 2.7% of cases. Only one of these three reports described the measurement of G-pH19). Watanabe et al.19) reported that the G-pH when LPZ was administered orally was significantly higher than without PPI administration. The G-pH was 5.5 or higher in all patients who received LPZ administration. However, G-pH was measured only on the day of ESD. The effects on G-pH before and after LPZ administration were not described in their report.
Among PPIs, rabeprazole sodium (RPZ) is known as a fast-acting PPI in terms of its acid-suppressive effect22). Inamori et al.23) reported that RPZ maintained the G-pH >3 and >4 longer than omeprazole at 6 hr after single-dose oral administration for Helicobacter pylori (Hp)-negative healthy volunteers. Because Hp-negative healthy volunteers have no atrophic gastric mucosa, most of their G-pH values are less than 2. However, the gastric mucosa of most gastric cancer patients is atrophied. For that reason, their G-pH is apparently higher than 4. Gursoy et al.24) reported that RPZ raised the mean G-pH 6.39 at 6 hr after single-dose oral administration for critically ill patients, such as trauma patients. In addition, RPZ is not susceptible to cytochrome P450 2C19 (CYP2C19) polymorphism25), although CYP2C19 is a kind of metabolic enzyme in PPI. Based on this fact, we presumed that RPZ would act quickly and that it would not differ among individuals. Consequently, we selected RPZ as PPI administered orally before ESD. We also would like to shorten oral RPZ administration to ESD to the greatest degree possible. Therefore, we conducted this study to evaluate the usefulness of oral RPZ administration on the day of ESD to prevent post-ESD bleeding with a gastric tumor compared with that administered three days before ESD.
This study enrolled 55 patients who underwent gastric ESD at Fukushima Medical University Hospital between November 2007 and November 2008. The indication for gastric ESD was well or moderately differentiated adenocarcinoma of any size without ulceration or sign of submucosal invasion. Even if biopsy of the lesion led to a diagnosis of adenoma, it was determined that ESD was indicated when it met one of the following criteria: more than 2 cm diameter, reddish color, depressed lesion, and increasing size. Patients were excluded if they had received acid-suppressive drugs up to one week before pre-ESD endoscopy or Hp eradication, or if they had a history of gastrectomy, major organ failure, or a drug allergy for PPI. This study was conducted with the approval of the Ethics Committee of Fukushima Medical University as number 585, and was registered in the university hospital Medical Information Network Clinical Trials Registry (UMIN-CTR) as number UMIN000011487. Informed consent was obtained from all patients in accordance with the institutional protocol.
ESD procedureESD was performed using a single-channel gastroscope (GIF-Q260J; Olympus Medical Systems Corp., Tokyo, Japan) and an electrosurgical unit (ICC-200; Erbe Elektromedezin GmbH, Tübingen, Germany). The procedure was conducted by two endoscopists, each with experience of more than 200 ESD procedures (T.H. and M.S.). As electrosurgical knives, FlexKnife (Olympus Medical Systems Corp., Tokyo, Japan) and ITknife2 (Olympus Medical Systems Corp., Tokyo, Japan) were used. Coagrasper (Olympus Medical Systems Corp., Tokyo, Japan) was used as electrosurgical hemostatic forceps. A mixture of hyaluronic acid (Artz; Kaken Pharmaceutical Co. Ltd., Tokyo, Japan, or MucoUp; Johnson & Johnson, Tokyo, Japan) and 10% glycerin plus 5% fructose in a 0.9% saline solution (Glyceol; Chugai Pharmaceutical Co. Ltd., Tokyo, Japan) containing 0.5% indigo carmine and 0.001% epinephrine was used to create a submucosal fluid cushion.
Study protocolAt our hospital, ESD is performed on Thursday and Wednesday each week. Patients are admitted the day before ESD. However, when the day before ESD is a holiday, patients must be admitted three days before ESD. Therefore, patients were assigned randomly to two groups, but according to their scheduled ESD: Group A patients were admitted on Thursday, with ESD performed on Wednesday; Group B patients were admitted on Friday, with ESD performed on Thursday. Group A patients would receive RPZ on the morning of ESD, whereas Group B patients would receive it beginning three days before ESD. For eight weeks, including on the day of ESD, 20 mg/day of RPZ was administered orally once a day, in the morning (Fig. 1). On the day of ESD, ESD was performed 7-8 hr after oral RPZ administration. Patients also fasted and received 2,000 ml/day of drip infusion intravenously, and did not receive PPI or H2RA administration intravenously for three days including the day of ESD. From the day after ESD, RPZ was again administered orally with water (Fig. 1). Patients were not administered gastric mucosal protective drugs or prokinetic drugs. Antiplatelets for other diseases were stopped from seven days before ESD to seven days after ESD. Warfarin as an anticoagulant was stopped from seven days before ESD. Then heparin was administered intravenously until the day of ESD. It was stopped 4-6 hr before ESD. After ESD, heparin was restarted immediately. The day after ESD, warfarin was restarted and heparin was stopped.
G-pH was measured when the endoscope was inserted into the stomach and was measured three times (Fig. 1): one month before ESD (pre-ESD pH), on the day of ESD (ESD pH), and seven days after ESD (post-ESD pH). ESD pH was measured immediately before ESD on 7-8 hr after oral RPZ administration. Post-ESD pH was measured 5-6 hr after oral RPZ administration. Patients drank no water for 3 hr before the procedure. Fifteen minutes before insertion of the gastroscope, 100 mg of viscous lidocaine solution (xylocaine 2%; AstraZeneca International, Osaka, Japan) was administered by inhalation as pharyngeal anesthesia. After insertion of the gastroscope, 1-2 ml of gastric juice was sampled using a catheter (PR-104: Olympus Medical Systems Corp., Tokyo, Japan) through a working channel in the endoscope. In addition, G-pH was measured using a pH sensor (Horiba Ltd., Kyoto, Japan). No anti-spastic drugs were used.

Study Protocol.
Patients were assigned to two groups receiving rabeprazole sodium (RPZ) beginning either 7-8 hr before ESD (Group A) or three days before ESD day (Group B). G-pH was measured three times (one month before ESD, immediately before ESD, and seven days after ESD).
The primary outcome was major bleeding after ESD for up to 56 days. Major bleeding was defined as hematemesis or melena that requires endoscopic hemostasis or depression of hemoglobin count by more than 2 g/dl14). A secondary outcome was changes in G-pH between Groups A and B, as measured on three separate occasions. Follow-up endoscopy was performed one week after ESD, and again after eight weeks.
Hp infection was determined from serum anti-Hp IgG antibody (Otsuka Pharmaceutical Co. Ltd., Tokyo, Japan). CYP2C19 genotyping was done using polymerase chain reaction — restriction fragment length polymorphism (PCR-RFLP) analysis. Based on point mutations in exon 4 and 5 of the CYP2C19 gene, individuals are classifiable into homo-extensive metabolizers (homEM), hetero-extensive metabolizers (hetEM), and poor metabolizers (PM).
Statistical analysisStatistical comparison of the patients was performed using the chi-square test for the bleeding rate. Patient characteristics within each group were analyzed using Student’s t-tests and chi-square tests. G-pH was expressed as a mean±standard deviation or median value. G-pH comparisons within each group were analyzed using the Wilcoxon single rank test, whereas comparisons of the G-pH between the two groups were analyzed using the Mann-Whitney U test. The G-pH between CYP2C19 polymorphism within each time of each group was analyzed using the Kruskal-Wallis test. Computer software (Statcel 2 OMS; Tokorozawa, Japan) was used for data analyses. The significance of differences was inferred for P values less than 0.05.
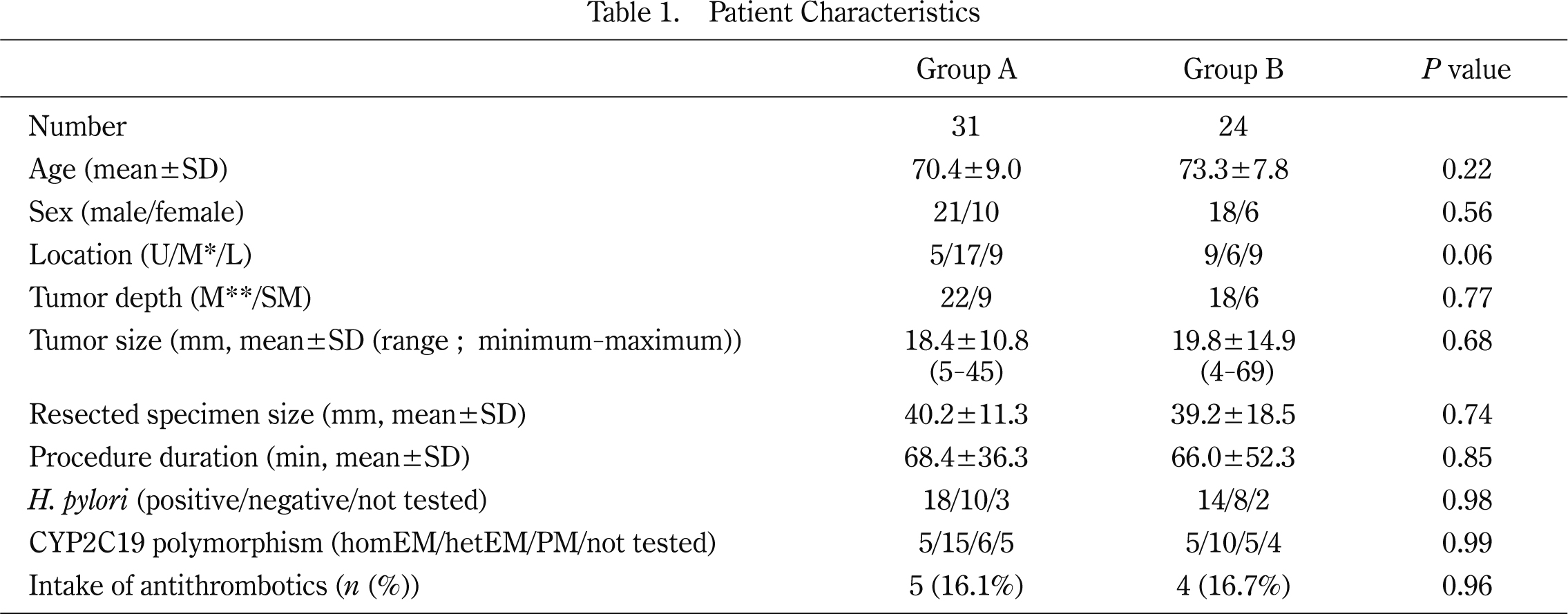
Of the 55 patients, 31 were in Group A and 24 were in Group B. The final diagnoses were 53 with adenocarcinoma and two with adenoma. Baseline data for the two groups are presented in Table 1. Mean age, gender, location of tumor, tumor depth, tumor size, resected specimen size, procedure duration, Hp infection rate, CYP2C19 polymorphism distribution, and intake of antithrombotics were not significantly different between the two groups. All antithrombotics were antiplatelets, not anticoagulants.
Patient Characteristics
U, upper stomach; M*, middle stomach; L, lower stomach; M**, mucosa; SM, submucosa; homEM, homo-extensive metabolizers; hetEM, hetero-extensive metabolizers; PM, poor metabolizers
Post-ESD bleeding was observed in 1.8% (1/55) of all the patients enrolled in this study: 3.2% (1/31) of the patients in Group A and 0% (0/24) in Group B. No significant difference was found between the two groups (P=0.37).
The post-ESD bleeding case was that of a 64-year-old man who had 11-mm-sized intramzucosal type 0-IIc well-differentiated adenocarcinoma in the anterior wall of the upper gastric body and 35-mm-sized post-ESD ulcer. He had not taken any antithrombotic before ESD. Seven days after ESD, although he had no symptom such as hematemesis or melena, he underwent endoscopy according to the study protocol. When the scope was inserted into the stomach, bleeding from a post-ESD ulcer was observed. Hemostasis was performed for the bleeding ulcer using the clipping method. Pre ESD-pH was 1.7 and ESD-pH was 7.0. Serum HP antibody was negative and CYP2C19 polymorphism was hetEM.
Effect of RPZ administration on raising G-pHAs shown in Table 2, the G-pH in Group A was 4.85±2.44, 6.53±1.11, and 6.87±1.46, respectively, in pre-ESD pH, ESD pH, and post-ESD pH. The ESD pH and the post-ESD pH were significantly higher than the pre-ESD pH in Group A (P=0.0055, P=0.00040, respectively). Similarly, the G-pH in Group B were 4.99±2.03, 7.20±0.58, and 6.92±1.45, respectively, in the pre-ESD pH, ESD pH, and post-ESD pH. Therefore, the ESD pH and the post-ESD pH were significantly higher than the pre-ESD pH (P=0.00013, P=0.00044, respectively). However, no significant difference was found between the ESD pH and post-ESD pH in either group (Group A, P=0.12; Group B, P=0.24). When comparing measured time, the ESD pH in Group A was significantly lower than in Group B (P=0.0048). However, no significant difference was found in the pre-ESD pH and post-ESD pH between the two groups. The G-pH between CYP2C19 polymorphism was also not significantly different among the times for any group (data not shown).

Comparison of Group A and B Gastric pH
In both groups, ESD pH and post-ESD pH were significantly higher than pre-ESD pH. The ESD pH in Group A was significantly lower than that in Group B. However, the ESD pH in Group A was higher than 6.
Data are shown as mean values±SD.
*vs. Pre-ESD pH: P<0.05.
No adverse event was observed as a result of administering RPZ orally.
In fact, RPZ, a fast-acting PPI in terms of its acid-suppressive effect22-24), is not susceptible to CYP2C19 polymorphism25). Therefore, we set two short periods (especially on the day of ESD) for preoperative administration of RPZ to examine its effect on post-ESD bleeding and on raising the G-pH for a gastric tumor. Patients were assigned to two groups. In Group A, oral RPZ administration before ESD was only 7-8 hr before ESD. From several previous reports22-24), we inferred that oral RPZ administration on 7-8 hr before ESD can raise G-pH >6.
With post-ESD bleeding, the total bleeding rate of all cases in this study was 1.8% (1/55). Only one case (3.2%) in Group A occurred post-ESD bleeding. However, his bleeding was found during follow-up endoscopy. He did not have hematemesis, melena, or anemia. Therefore, oral RPZ administration at least 7 hr before ESD as well as three days before ESD contributed to the prevention of post-ESD bleeding because the post-ESD bleeding rate was low. No significant differences were found between the two groups for post-ESD bleeding. With the effect of RPZ administration on raising G-pH, the ESD pH was significantly higher than the pre-ESD pH in both groups. The ESD pH in Group A was significantly lower than in Group B. However, because the ESD pH in Group A remained higher than 6 (6.53), the protocol used for Group A was also effective for acid suppression. The post-ESD pH, which remained higher than 6, was not significantly different between the two groups. Beginning oral RPZ administration three days before ESD might be more effective than that on the day of ESD because the ESD pH in Group A was significantly higher than in Group B. However, oral RPZ administration starting on the day of ESD, as well as three days before ESD, was sufficiently effective in suppressing gastric acid to prevent post-ESD bleeding.
This study was the first in the world conducted to evaluate changes of G-pH resulting from oral PPI administration before gastric ESD. Oral RPZ administration is apparently simpler and easier and cheaper than intravenous PPI administration as the route of administration. The results of this study suggest that intravenous PPI administration was not necessary to prevent post-ESD bleeding if oral RPZ was administered at least 7 hr before gastric ESD. In conclusion, oral RPZ administration at least 7 hr before ESD provides a sufficient gastric acid-suppressive effect during and after ESD to prevent post-ESD bleeding in most gastric tumor cases. However, this study was limited by the fact that it was conducted at a single center and also by the fact that few patients were enrolled. An additional study is planned with incorporation of a multicenter trial with a more detailed breakdown of the effect of oral PRZ administration in the days before ESD.
We would like to express our gratitude to Ms. Kyoko Onuma and Ms. Chikako Sato for their collaboration in the measurement of CYP2C19 polymorphism and to all endoscopy staff for their collaboration in assistance of endoscopic procedures, including ESD.
The authors declare no conflicts of interest for this article.