2018 年 64 巻 2 号 p. 82-88
2018 年 64 巻 2 号 p. 82-88
Lymphoproliferative disorder (LPD) is a potentially severe adverse effect of methotrexate (MTX) administration in patients with rheumatoid arthritis (RA). We report a case of MTX-associated LPD (MTX-LPD) in a patient with RA who developed severe pulmonary failure complicated by perforation of the terminal ileum. A 61-year-old woman with RA receiving MTX complained of dyspnea and abdominal pain. She was diagnosed with intestinal perforation and peritonitis, and underwent immediate abdominal surgery. Pathological examinations of the specimen obtained from the resected ileum and a bone marrow aspirate revealed diffuse large B-cell lymphoma. Steroid therapy failed to improve her respiratory failure, but her condition improved after abdominal surgery and suspension of MTX. MTX-LPD can result in multiple life-threatening conditions; however, the symptoms are highly variable. RA patients receiving MTX should thus be monitored carefully, and MTX administration should be stopped immediately on suspicion of MTX-LPD.
Methotrexate (MTX) is a key drug in the treatment of rheumatoid arthritis (RA), sometimes referred to as an “anchor drug”. A 2016 update to the European League Against Rheumatism recommendation on RA stated that MTX should be used after the initial diagnosis of RA, if no contraindications for this drug were present1). The 2015 American College of Rheumatology Guidelines for the treatment of RA likewise state that disease-modifying antirheumatic drug-naïve patients with established RA should receive such an agent, with MTX as the preferred choice2). However, Anderson et al. reported a significantly increased risk of non-Hodgkin lymphoma in a cohort of American RA patients receiving MTX (odds ratio, 1.2; 95% confidence interval, 1.1-1.3)3), while lymphoproliferative disorder (LPD) has also been reported in RA patients administered MTX4-7). LPD induced by MTX (MTX-associated LPD; MTX-LPD) is categorized as an other iatrogenic immunodeficiency-associated (OIIA) LPD in the 4th edition of the World Health Organization classification of tumors of hematopoietic and lymphoid tissues8). Stopping MTX has been reported to result in a complete response (CR) in 40%-60% of patients with MTX-LPD, with no need for any additional medications9,10). In addition, approximately 40%-50% of MTX-LPD occurs at extranodal sites, including the gastrointestinal tract, skin, liver, lungs, and kidneys5,11).
We report a patient with RA treated with MTX who developed severe pulmonary failure complicated by perforation of the terminal ileum. Histological examination showed LPD around the perforated region in the terminal ileum. The patient’s LPD and respiratory condition improved after partial resection of the perforated ileum and suspension of MTX administration.
A 66-year-old woman was admitted to our hospital with severe dyspnea. She had developed arthritis of her bilateral wrist joints 7 years earlier, and had been diagnosed with RA. She was initially administered bucillamine with no improvement in her wrist pain, but her joint pain was relieved after the addition of MTX 6 mg/week. Pain in her bilateral knee joints worsened 5 years after her initial diagnosis but was controlled by increasing the MTX dose to 12 mg/week. On admission, her axillary temperature was 37.2°C, blood pressure was 125/69 mmHg, and oxygen saturation was 90% under oxygen inhalation at 10 L/min. There were no palpable swollen lymph nodes on physical examination, and no evident skin ulcers or rashes. Fine crepitations were audible by chest auscultation over both lungs. No swollen or tender joints were identified in the upper or lower extremities. Laboratory findings were as follows: white blood cell count, 22,700/μL (neutrophils, 72%; monocytes, 4.5%; lymphocytes, 8.5%; atypical lymphocytes, 2.5%); hemoglobin, 12.8 g/dL; platelet count, 81,000/μL; total protein, 4.9 g/dL; albumin, 2.8 g/dL; lactate dehydrogenase, 545 U/L; C-reactive protein, 6.11 mg/dL; β-D-glucan, < 6.0 pg/mL; and soluble interleukin-2 receptor, 11,000 U/mL. Chest X-ray and computed tomography (CT) of the neck, chest, and abdomen showed severe and extensive interstitial shadows in bilateral lung fields, but no lymphadenopathy (Fig. 1a, b). Acute exacerbation of interstitial pneumonia was diagnosed, with MTX-induced interstitial pneumonia, Pneumocystis jirovecii pneumonia, or RA-related lung complications as possible causes. MTX administration was therefore suspended on hospital day 1, and the patient was treated with methylprednisolone pulse therapy at 500 mg/day and trimethoprim-sulfamethoxazole combination at 9 g/day for 3 days. Respiratory management was initiated using noninvasive positive-pressure ventilation due to low oxygen saturation. However, the patient’s respiratory condition failed to improve, despite these measures. On hospital day 4, the patient complained of worsening abdominal pain after eating some jelly. A chest X-ray the following day showed free air under the right diaphragm, and CT showed massive abdominal effusion and free air in the abdominal cavity (Fig. 1c). Perforation of the digestive tract and peritonitis were diagnosed and immediate abdominal surgery was performed. The perforation site was identified as the terminal ileum (about 30 cm oral to the ileocecal region) (Fig. 2a), and the patient underwent partial excision around the perforated region and intraperitoneal cleaning drainage.
Histological examination of the resected ileum and a bone marrow aspirate obtained on hospital day 3 were performed. The resected ileum showed numerous lymphoid cells around the perforated region, comprising small to middle-sized CD3-positive cells mixed with large CD20-positive B cells. Immunostaining revealed CD30-positive and latent membrane protein 1-positive cells with similar distributions to the CD20-positive B cells. Several Epstein-Barr virus-encoded small RNA (EBER)-positive cells were observed in this histological sample (Fig. 2b-d). The bone marrow showed accumulation of atypical lymphoid cells, including CD8-positive T cells, CD20-positive B cells, and small numbers of EBER-positive cells. The patient was diagnosed with OIIA LPD according to the World Health Organization classification, specifically MTX-LPD, with a histological diagnosis of diffuse large B-cell lymphoma (DLBCL), Ann Arbor stage IVA.
Prednisolone 40 mg/day was administered postoperatively for 7 days, and the patient’s respiratory condition improved after extubation. Her condition remained good after gradual tapering of the prednisolone, with almost normal appearances of both lung fields on chest X-ray and CT, despite no specific treatments for LPD (Fig. 3). The prednisolone dose was tapered to 15 mg/day on day 40, and the patient was discharged from hospital on day 46 (Fig. 4). The patient’s condition remained good after discharge. Whole-body CT revealed no findings associated with recurrence of LPD, and she was thus considered to have achieved CR, although no re-examination of bone marrow aspirate was performed.
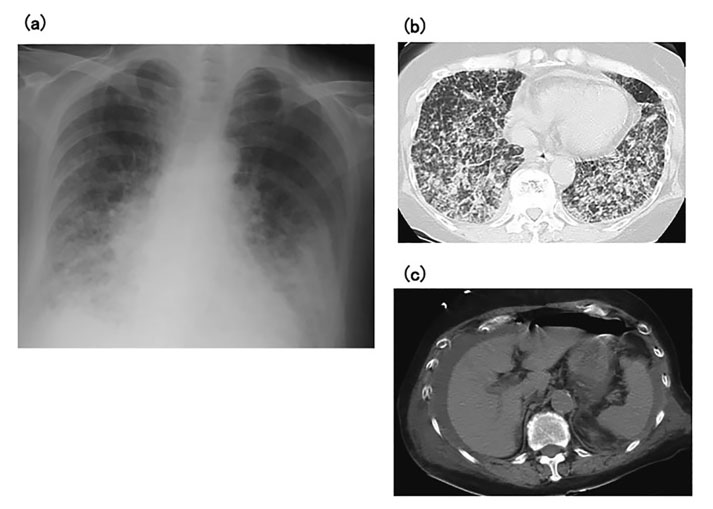
(a) Chest X-ray on admission shows reticular shadows in both lungs. (b) Chest computed tomography on admission shows extensive interstitial shadows in both lungs, particularly in the lower lung fields. (c) Chest computed tomography on hospital day 4 shows a water-density mass around the liver and free gas in the abdominal cavity.
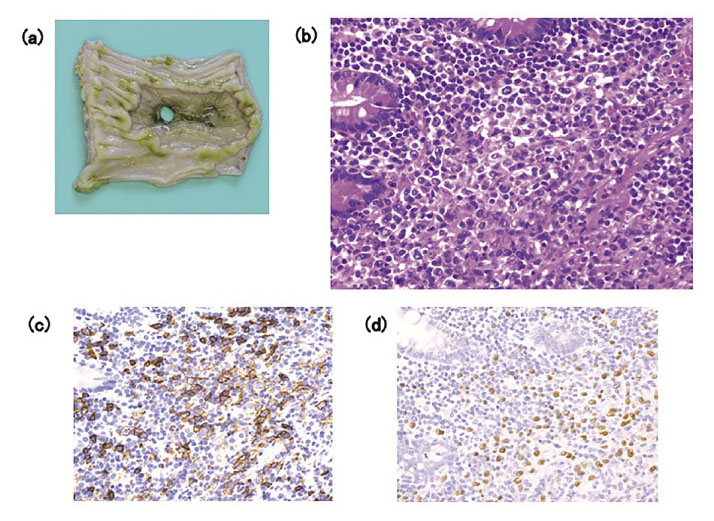
(a) Macroscopic view of the site of perforation in the terminal ileum (about 30 cm oral to the ileocecal region). (b) Histological examination shows numerous atypical lymphoid cells around the perforated region of the ileum (hematoxylin and eosin staining, ×400). (c) The infiltrated lymphoid cells include large CD20-positive B cells (CD20 immunostaining, ×400). (d) Several cells show positive staining for Epstein-Barr virus-encoded small RNA (×400).
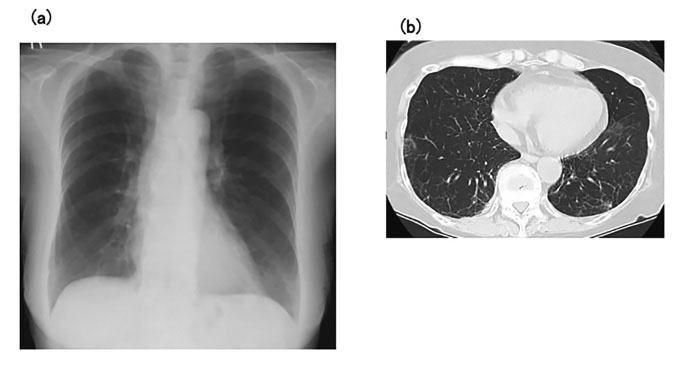
(a) Chest X-ray at discharge shows almost normal results for both lungs. (b) Chest computed tomography at discharge shows disappearance of abnormal shadows from the lung fields.

Clinical course of the patient.
NPPV, noninvasive positive-pressure ventilation; MEPM, meropenem; TAZ/PIPC, tazobactam/piperacillin; ST, sulfamethoxazole-trimethoprim; PSL, prednisolone; CRP, C-reactive protein; LD, lactate dehydrogenase; Ly, lymphocytes.
We describe a patient with MTX-LPD who presented with two characteristic features: acute peritonitis due to perforation of the ileum attributed to MTX-LPD, and respiratory failure that improved after partial excision of the perforated region and prednisolone administration, despite steroid therapy and antibiotics being ineffective before surgery.
Regarding ileal perforation, Hoshida et al. reported 76 cases of RA-associated LPD, identifying the primary site as nodal in 34 and extranodal in 35 patients, including two patients with LPD affecting the terminal ileum5). Among 48 patients with MTX-LPD, the primary site was nodal in 22 and extranodal in 23 patients5). Thirty-eight of the 48 MTX-LPD patients and 60 of the 76 RA-associated LPD patients had B cell immunophenotype5). Five other cases of MTX-LPD complicated by perforation of the intestine and peritonitis have been reported in the Japanese literature and are summarized in Table 112-16). All these patients were Japanese and four of the cases were reported by surgeons. In all cases, including the current case, MTX-LPD was diagnosed at the same time as intestinal perforation, and treatment for LPD was therefore not provided. The mean age at onset of MTX-LPD was 73.5 years (range, 63-87 years), the mean duration of MTX administration at diagnosis was 3.8 years (range, 2-7 years), and the mean maximum weekly dosage of MTX was 7.9 mg/week (range, 4-12 mg/week). The site of perforation was the distal ileum and pathological examination of the perforation site showed diffuse large B-cell lymphoma in all patients. All four patients examined for EBER showed positive results. Five of the six patients (including the current case) were treated with surgery alone, while one patient also received chemotherapy. Five patients achieved CR but the remaining patient died of pneumonia. Although the reported cases were generally treated successfully, the findings suggest that most cases of MTX-LPD complicated by intestinal perforation might involve EBER-positive DLBCL, and CR may be achievable by simply stopping MTX administration and resecting the perforated region, as in our patient. Ichikawa et al. reported spontaneous improvement in 59% of MTX-LPD patients after MTX withdrawal, associated with Epstein-Barr virus positivity and non-DLBCL status7). Although all reported cases of MTX-LPD complicated by intestinal perforation, including the current patient, showed DLBCL on histological examination, their clinical outcomes were generally good. The reasons for the good outcomes in patients with this type of MTX-LPD are unclear. Importantly, we must consider the bias caused by the fact that only successful cases tend to be reported. However, it is also possible that the evident symptoms of intestinal perforation, such as abdominal pain, and the availability of histological specimens because most patients with intestinal perforation undergo surgery, may contribute to the prompt diagnosis and treatment of LPD. Tokuhira et al. reported LPD regression after MTX withdrawal in 43 of 63 MTX-LPD patients, including 26 patients with stage IV17). Such findings support the withdrawal of MTX-LPD without additional chemotherapy, even in patients with high clinical stage, such as the current case. In contrast, Vaidya et al. reported that 14 of 50 patients with malignant lymphoma who developed intestinal perforation died due to perforation18). This suggests that intestinal perforation may not be a good prognostic phenomenon in patients with MTX-LPD. The main cause of intestinal perforation in patients with malignant lymphoma is fragility of the intestinal wall due to infiltration of lymphoma cells. About 80% of the gross morphology in cases of malignant lymphoma with intestinal perforation was reportedly ulcer type19). Some patients with malignant lymphoma underwent chemotherapy and experienced intestinal perforation due to reduction of the lesion18). The current patient received high-dose prednisolone before developing intestinal perforation, and we could therefore not exclude the possibility that this administration of high-dose steroids contributed to their intestinal perforation.
Saito et al. and Inui et al. reported that a significant decrease in absolute lymphocyte count at LPD diagnosis and its restoration after MTX withdrawal were associated with spontaneous regression of LPD that developed during MTX treatment20,21); however, Takanashi et al. reported opposing results22). Further research is therefore needed to determine if a rapid recovery of absolute lymphocyte count after MTX withdrawal represents a predictive factor for LPD regression. In our patient, the absolute lymphocyte count before the onset of MTX-LPD was low (627/μL), but the recovery of lymphocyte count after MTX withdrawal was difficult to evaluate because the patient was administered high-dose prednisolone and developed complications of severe peritonitis. However, 6 months before developing MTX-LPD, she had experienced high-grade fever for several weeks and her absolute lymphocyte count at that time was 342/μL. Her lymphocyte count recovered immediately after temporary withdrawal of MTX, to 1,641/μL for 4 days. This pattern may thus support the previous reports.
Regarding the occurrence of respiratory failure, few reports have described MTX-LPD of the lung, and lung lesions in patients with MTX-LPD thus remain to be characterized adequately. Hoshida et al. reported only four patients with the lung as the primary site among 48 patients with MTX-LPD5), while Shimizu et al. reported eight MTX-LPD patients with lung abnormalities, representing tumors or nodules, but no interstitial change23). Kameda et al. reported RA patients receiving MTX therapy who developed both lymphomatoid granulomatosis and interstitial pneumonia with pathological features of diffuse alveolar damage24), but included only one report of an MTX-LPD patient with interstitial pneumonia. They suggested three probable causes of interstitial pneumonia in this patient: RA disease activity; an adverse event associated with MTX administration; or lymphomatoid granulomatosis of the lung. In another rare case of a patient with interstitial pneumonia associated with malignant lymphoma of the mediastinum treated by chemotherapy, the malignant lymphoma and the interstitial lung disease improved simultaneously25). We initially considered that the severe respiratory failure in our patient could have been attributable to P. jirovecii pneumonia, MTX-induced lung injury, or RA involvement of the lungs. Administration of MTX was therefore suspended and high-dose prednisolone and trimethoprim-sulfamethoxazole combination were administered, but the patient’s respiratory condition failed to improve. However, her lung condition improved relatively rapidly after surgery. We were unable to perform histological examination using bronchoscopy in the current patient because of her poor general condition, and we could therefore not rule out the possibility that the MTX-induced interstitial pneumonia had been improving slowly under high-dose steroids. However, her clinical course suggested that the lung lesions were likely to represent reactive changes associated with MTX-LPD.
In conclusion, we encountered a patient with MTX-LPD complicated by peritonitis due to perforation of the ileum, and severe respiratory failure due to interstitial pneumonia. Her condition improved after withdrawal of MTX and surgery. This case highlights the need to be aware of the risk of MTX-LPD when administering MTX to RA patients. Further reports should be accumulated to determine the predictive factors for MTX-LPD.
Potential conflicts of interest: None.

Patients with methotrexate-associated lymphoproliferative disorder complicated by intestinal perforation
RA, rheumatoid arthritis; MTX, methotrexate; EBER, Epstein-Barr virus-encoded small RNA; Ref., reference; M, male; F, female; ND, no data; DLBCL, diffuse large B-cell lymphoma; CT, chemotherapy; CR, complete response
We thank Susan Furness, PhD, from Edanz Group (www.edanzediting.com/ac) for editing a draft of this manuscript.