2018 年 64 巻 2 号 p. 89-94
2018 年 64 巻 2 号 p. 89-94
Anti-melanoma differentiation-associated gene 5 (MDA5) antibody-positive clinically amyopathic dermatomyositis (CADM) is frequently associated with rapidly progressive interstitial lung disease (RP-ILD) resulting in high mortality. Here we report a 51-year-old Japanese woman with anti-MDA5 antibody-positive hypomyopathic dermatomyositis (DM) who developed RP-ILD. She developed respiratory failure and pneumomediastinum, however her RP-ILD responded favorably to the combined immunosuppressive treatments consisting of steroids, intravenous cyclophosphamide and tacrolimus. She was complicated with severe infections, which were successfully managed by combined modality therapy including artificial ventilation and antibiotics in addition to immunosuppressive treatments in parallel to the decline of anti-MDA5 antibody titer (>150 Index to 75 Index). She was discharged after 6 months of treatment without any respiratory sequelae. Hypomyopathic DM patients with high titers of anti-MDA5 antibody should be treated with aggressive immunosuppressive therapies and closely monitored to prevent various infections.
Clinically amyopathic dermatomyositis (CADM)/ hypomyopathic dermatomyositis, a subtype of dermatomyositis (DM) with subtle muscle involvement, can be accompanied by rapidly progressive interstitial lung disease (RP-ILD), which is a life-threating complication1). Detection of antibody against CADM-140/melanoma differentiation-associated gene 5 (MDA5) is diagnostic of CADM2). Furthermore, CADM patients with anti-MDA5 antibodies often have accompanying RP-ILD, which is associated with a high mortality rate3). Moreover, the correlation between the titer of anti-MDA5 antibodies and clinical course has been demonstrated in CADM patients4). Cytokine storms may contribute to the pathogenesis of RP-ILD with anti-MDA5 antibody5), however, the precise mechanism remains unknown. Previous reports suggest that the presence of anti-MDA-5 antibodies represents an individual risk factor for death from ILD in DM patients6) and aggressive immunosuppression could be vital despite increasing the risk of severe infections. Here we report a case of anti-MDA5 antibody-positive hypomyopathic DM complicated with RP-ILD and pneumomediastinum, which was resistant to high-dose steroids but was treated successfully with combined immunosuppressive agents including tacrolimus. We also show reduction in anti-MDA5 antibody levels in line with the improvement of RP-ILD following these immunosuppressive therapies.
A 51-year-old Japanese woman was previously admitted to hospital presenting with skin rash and proximal muscle weakness, following referral for examination of rheumatic diseases by a dermatologist. Eight weeks before admission, she noticed palmar erythema and felt difficulty in climbing stairs. Gottron’s papules, inverse gottron’s papules and periungual erythemas were found (Figure 1, 2). There was no dry cough or shortness of breath. Laboratory findings showed slightly elevated creatine kinase (275 U/L) and myoglobin (72 ng/mL). Magnetic resonance imaging (MRI) showed slight high intensity of the right rectus femoris. She was diagnosed with DM and admitted to the hospital. Anti-MDA5 antibody was positive (>150 Index), however, computerized tomography (CT) showed no ILD (Figure 3). Systemic detailed examination showed no finding of malignancies. She was initially treated with methylprednisolone (mPSL) (125 mg, 3 days), followed by oral prednisolone (PSL, 60 mg/day). Two weeks after initiation of steroid, she developed dysphagia and was treated with mPSL pulse (500 mg, 3 days). Two weeks after steroid pulse therapy, the patient suffered respiratory failure. Chest computed tomography (CT) showed consolidation and ground-glass opacity of the whole pulmonary field area (Figure 4) and she was given a diagnosis of RP-ILD. Repeated intravenous pulse therapy (mPSL 1 g) was administered and she was transferred to our hospital.
On admission, her oxygen saturation was 77% using 10 L/min oxygen administration. A physical examination revealed bilateral wheezes, purpura on the extensor side of the elbows and palmar erythema. An arterial blood gas analysis showed a pH of 7.519, PaO2 of 39.9 mmHg, and PaCO2 of 29.4 mmHg. Laboratory data (Table 1) revealed mild liver dysfunction (alanine transaminase, 86 U/L and lactate dehydrogenase, 436 U/L), white blood cell (WBC) count of 3,500/μL (neutrophils, 93.0%; monocytes, 2.0%; lymphocytes, 5.0%), and elevated levels of C-reactive protein (5.3 mg/dL) (Table 1). The creatine kinase, aldolase and myoglobin levels were increased at 243 U/mL, 8.3 U/L and 180 mg/dL, respectively. Anti-SS-A antibody was positive (19.0 U/mL). In terms of ILD markers, the level of KL-6 was 560 U/mL, surfactant protein (SP)-A was 195.0 ng/mL and SP-D was 207.9 ng/mL.
Noninvasive positive pressure ventilation was started. We selected combination therapy, including high-dose mPSL 1 g pulse for 3 days, followed by 80 mg/day of PSL and intravenous cyclophosphamide (IV-CY; 500 mg/m2). Six days after IV-CY therapy, leukocytopenia occurred (WBC 1,800/μL, Ly 54/μL) and the patient was treated with granulocyte-colony stimulating factor. With these treatments, the creatine kinase and myoglobin levels were decreased after the 14th day. On the other hand, pneumomediastinum was further complicated with RP-ILD (Figure 5 a, d) and her respiratory failure progressed and oral intubation with mechanical ventilation was introduced on the 8th day of hospitalization.
We administered cyclosporine A (CyA) on the 22th day, which was discontinued due to severe leukocytopenia (WBC 300/μL, Ly 10/μL) on day 30. During these immunosuppressive treatments, she was complicated with severe infections, including candidemia, aspiration pneumonia and herpes zoster, which were successfully managed by Fluconazole, Tazobactam/ Piperacilin and acyclovir.
Mediastinal emphysema disappeared but new ground-glass opacity appeared on CT at day 60 (Figure 5 b, e). We started tacrolimus on 80th day which was increased finally up to 4 mg, while PSL was tapered. Following 2 months of therapeutic courses, the patient gradually improved, oxygen requirement decreased and she was withdrawn from artificial ventilation. Six months later, the anti-MDA5 antibody titer had decreased to 75 Index. The serum ferritin level increased to a maximum value of 975 ng/mL on day 16, which also declined in line with clinical improvement. The patient was transferred to another hospital for rehabilitation (Figure 5 c, f). Ultimately, she returned home with domiciliary oxygen therapy (1 L/min) and remains under treatment as an outpatient at our hospital (Figure 6).
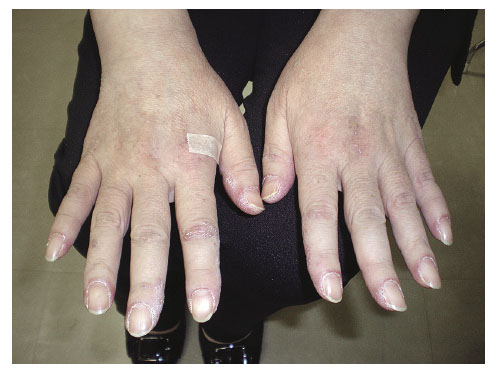
Skin lesions squamosal erythema on the dorsum of the hand and periungual erythemas.

Skin lesions of the hand showing palmar papules and erythema keratodes.
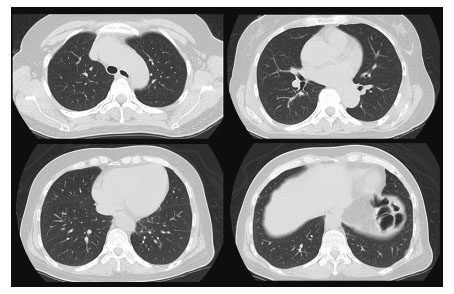
No findings of interstitial lung disease were found on chest computed tomography (CT) at the first admission in the previous hospital.

Chest CT showed bilateral consolidation and ground-glass opacity (GGO) in both lungs when the patient transferred to our hospital.
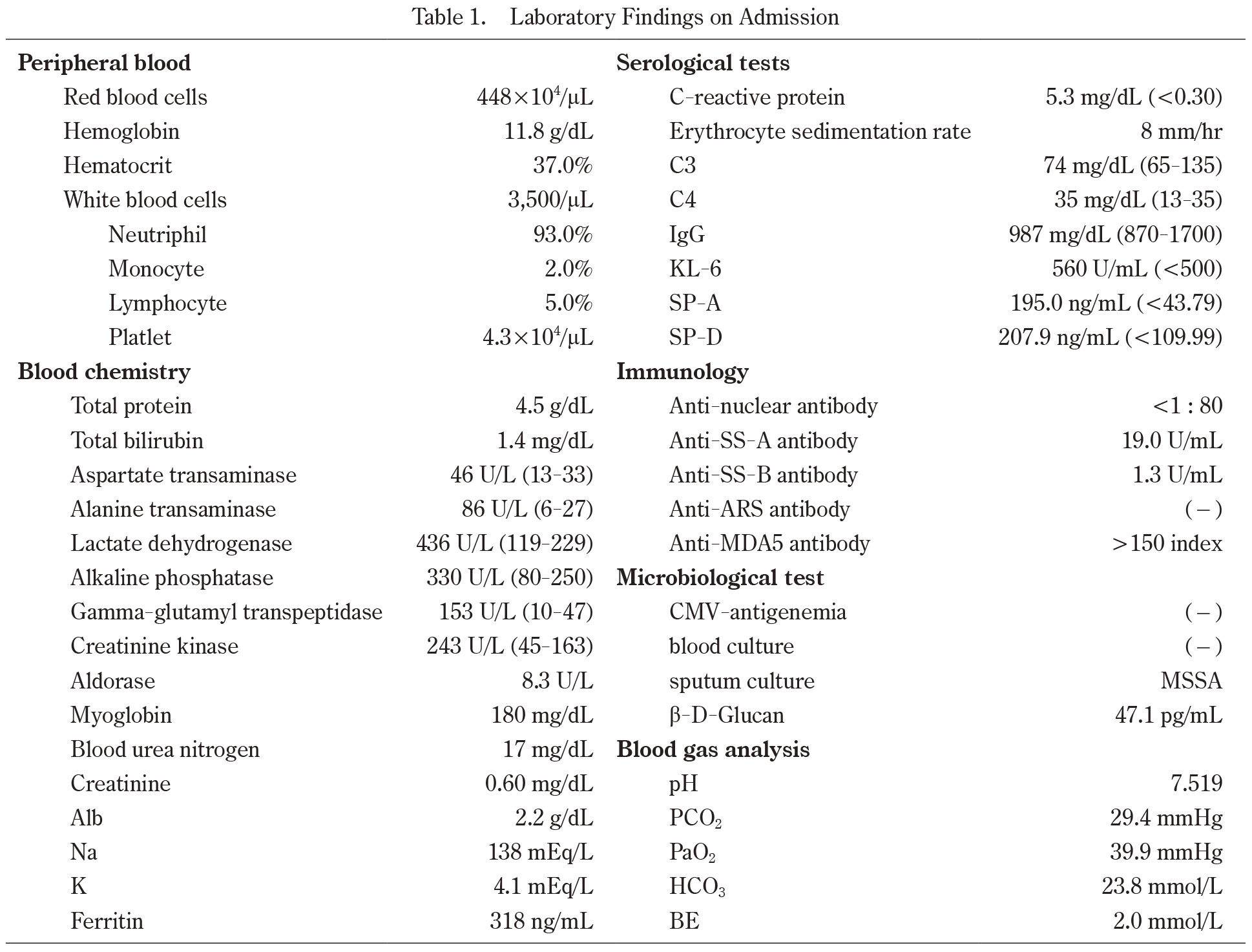
Laboratory Findings on Admission
Abbreviation: CMV; cytomegalovirus, HBsAg; hepatitis B surface antigen, HCV; hepatitic C virus, SPA; surfactant protein A, SPD; surfactant protein D

Time course of chest CT images.
(a,d) Pneumomediastinum, consolidation and patchy GGO were shown in both lungs. (b,e) Pneumomediastinum was disappeared 2 months after immunosuppressive treatment. GGO and consolidation were still showed bilateral lobes.
(c,f) CT showed lung fibrosis mainly in the left lobe, but consolidation and GGO dramatically improved 1 year after immunosuppressive treatment.

Clinical course of the present patient.
CADM, a subtype of DM with subtle or no muscle involvement, is occasionally accompanied by fatal, RP-ILD that is resistant to immunosuppressive therapy7). Previous observations suggested that high titers of anti-CADM-140/MDA5 autoantibodies predict a more severe disease course and resistance to immunosuppressive therapy in patients with CADM8). We describe a patient with hypomyopathic DM who developed RP-ILD with a high titer of anti-MDA5 autoantibodies. The condition was resistant to high dose steroid, but was successfully treated with combined immunosuppressive therapy including PSL, tacrolimus and IV-CY despite refractory respiratory failure and pneumomediastinum, which were complicated by RP-ILD.
It has been debated whether aggressive immunosuppressive therapy improves the prognosis of patients with CADM9). Previous observations suggested that high titers of anti-CADM-140/MDA5 autoantibodies confer a high risk for the development of RP-ILD in several cohorts4,10). The administration of intensive immunosuppressive therapy prior to irreversible pulmonary damage might improve the prognosis of RP-ILD in patients with DM. Recently it has been demonstrated that early induction of combined immunosuppressive therapy consisting of high dose corticosteroids, IV-CY and calcineurin inhibitors may improve survival of DM patients with anti-MDA-5 positivity11). There is evidence that the related calcineurin inhibitor, tacrolimus, may be superior to CyA12); Ando et al. reported patients with refractory DM-associated ILD despite steroid and CyA therapy, who demonstrated a prompt response to tacrolimus13). The pharmacological potency of tacrolimus is 100 times greater than that of CyA14). Furthermore, it is reported that the shift from CyA to tacrolimus induce down-regulation MCP-1 production in BALF15), resulted in reducing the lung fibrosis16). Therefore, we introduced these immunosuppressive treatments against anti-MDA-5 antibody-positive DM patients with RP-ILD, despite the risk of severe infections.
Generally, these treatments are associated with infections, including opportunistic infections due to profound immunosuppression17). In the present case, such urgent strong immunosuppressive therapy successfully resulted in clinical improvement of RP-ILD despite the complication of severe infections, which were successfully managed with antibiotics. Gono et al. reported that high levels of serum ferritin were associated with the development and prognosis of RP-ILD with DM, including those with anti-MDA5 antibodies18). Indeed, high levels of ferritin and anti-MDA-5 titers seem to correlate with treatment response of RP-ILD in the present case. It was demonstrated that the anti-MDA5 antibody titer before treatment was not predictive of the prognosis of RP-ILD in anti-MDA5 antibody-positive DM19). On the other hand, the anti-MDA5 antibody titers declined in improving patients who survived4). Therefore, serum levels of anti-MDA5 antibody could be useful for evaluating the response to treatment or deciding upon therapeutic strategy in anti-MDA5-positive DM patients with ILD.
Although pneumomediastinum in patients with myositis has been rarely reported, the vast majority of cases have occurred in patients with DM20). Spontaneous pneumomediastinum complicated with DM is associated with results in significant morbidity and mortality21). The pathogenesis of pneumomediastinum in patients with myositis is poorly understood, though it is speculated to result from the rupture of subpleural blebs that occur with ILD, leading to dissection of air around perivascular sheaths and into the mediastinum22). Pneumomediastinum tends to occur with RP-ILD and should be considered as a serious pulmonary complication of anti-MDA5 antibody-positive DM.
In conclusion, early aggressive therapy is necessary because anti-MDA5 antibodies are associated with high mortality in DM patients with RP-ILD. When DM patients are encountered with high anti-MDA5 titers, early induction of combined strong immunosuppressive therapy may serve to improve their prognosis despite the risks of severe infections. Proper monitoring, prevention and prompt management of infections are also necessary for the treatment of RP-ILD associated with hypomyopathic DM.
A written informed consent for this case report has been obtained from the patient.
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.