Abstract
Since the development of endoscopic ultrasound-guided fine needle aspiration (EUS-FNA) in the early 1990s, its application has been extended to various diseases. For pancreatic cancer (PC), EUS-FNA can obtain specimens from the tumor itself with fewer complications than other methods. EUS-FNA can also be more useful for TNM staging than other imaging modalities. Furthermore, EUS-FNA can contribute to precision medicine by obtaining tissue for immunohistochemical or genetic studies from primary or metastatic sites of diseases. This paper will focus on the role of EUS-FNA in PC.
Introduction
Pancreatic cancer (PC) is associated with a very poor prognosis, highlighted by the close parallel between disease incidence and mortality2). Five-year survival in patients with PC remains as low as 6% in the USA3,4). EUS-FNA was established in the early 1990s and is now considered one of the standard procedures for PC diagnosis1). Its applications have been widening from tissue sampling to disease staging. This article presents a review of several recent developments in EUS-FNA in PC.
Diagnosis of PC using EUS-FNA
Our group previously reported the superiority of EUS-FNA to endoscopic retrograde pancreatography (ERP) in the diagnosis of PC without biliary stricture5). In this study, we included 83 patients (EUS-FNA in 53 patients and ERP in 30 patients) and found that EUS-FNA showed sensitivity of 92.9% and accuracy of 94.3%, while ERP showed sensitivity of 33.3% and accuracy of 46.7%. With regard to complications, there was a significant difference (P<0.01) in the frequency of post-procedure pancreatitis between the EUS-FNA group and the ERP group (0%, 0/53 vs 33.3%, 10/30, respectively). Considering these advantages of EUS-FNA, EUS-FNA now plays an important role in the diagnostic algorithm of PC6).
Regarding the diagnostic yield of EUS-FNA for PCs, several retrospective and prospective studies have been published. These series show significant variability in the reported sensitivity and specificity of EUS-FNA. To summarize available evidence on the diagnostic accuracy and safety of EUS-FNA for solid lesions of the pancreas, Hewitt et al. conducted a meta-analysis that included 33 studies published between 1997 and 2009 with a total number of 4984 patients7). The pooled sensitivity for malignant cytology was 85% (95% confidence interval [CI], 84-86), and the pooled specificity was 98% (95% CI, 97-99). Variables that appeared to affect diagnostic yield were prospective study design and multicenter study7).
Another factor that can improve the diagnostic yield of EUS-FNA is the presence of an on-site cytopathologist who can evaluate the quality and quantity of the obtained specimen (rapid on-site evaluation: ROSE)8-10). ROSE is now considered a standard method for EUS-FNA because it not only improves the diagnostic value, but it also reduces the complication rate. However, some centers do not have this capability because of the cost and a lack of resources. Considering this challenging situation, we conducted a prospective study to evaluate the optimal number of needle passes with a 25-gauge needle for solid pancreatic lesions (SPLs) without ROSE. This study was a preliminary study with 20 patients in each group (Group A:EUS-FNA with 4 needle passes, Group B: EUS-FNA with ROSE). We found that the sampling rate was higher in Group B (20/20, 100%) than in Group A (19/20, 95%), but there was no significant difference between them (P-value = 0.31). In Group A, sensitivity, specificity and accuracy were 100% among the 19 cases. In Group B, sensitivity was 94.1%, specificity was 100%, and accuracy was 95%. There were also no significant differences between the groups. No complications were seen. Our study suggests that four needle passes using a 25-gauge needle may be sufficient for EUS-FNA of SPLs where onsite cytology is not available11). Subsequent studies with the same objectives have confirmed our findings12,13).
EUS-FNA for pancreatic cancer staging
Staging of PC is done according to the American Join Committee of Cancer (AJCC) and the Union for International Cancer Control (UICC) Staging TNM classification, which describes tumor extension (T) and lymph node (N) and distant metastases (M). Reported accuracies of T-staging by EUS range from 62-94%, and those of N-staging by EUS range from 50-86%. Additionally, EUS-FNA can contribute to accurate TNM staging on some special occasions14-20).
1. Peritoneal tumor dissemination or malignant ascites
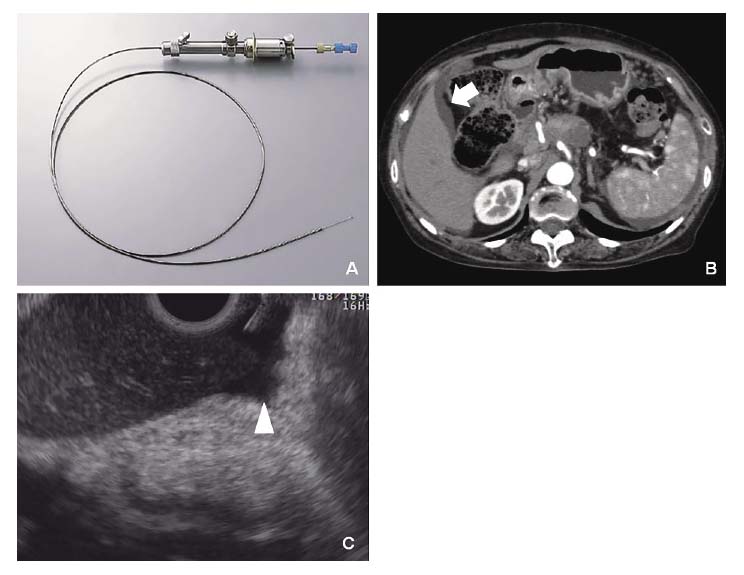
Accurate diagnosis of peritoneal tumor dissemination or malignant ascites in cancer patients is required to select proper treatment options. However, it is occasionally difficult to detect or obtain a minute amount of ascites by conventional modalities. EUS can detect a minute or minimal amount of ascitic fluid that may be undetectable with other imaging modalities, including abdominal ultrasound (US), computed tomography (CT), and magnetic resonance imaging (MRI). Moreover, EUS-guided abdominal paracentesis (EUS-P) has the potential to play an important role for staging of cancer since the establishment of malignant ascites denotes a more advanced stage of cancer21-23). Although EUS-P is a useful technique at times, we encountered technical difficulties during EUS-P, probably due to a weaker counteracting force from extramural objects and a lax gastrointestinal wall. We previously reported the usefulness of a spring-loaded needle device for EUS-P in 11 patients with known malignancies (6 PCs). Our results showed that EUS-P with an automated spring-loaded needle device can be a useful technique to obtain a minute amount of ascitic fluid in cancer patients. Furthermore, EUS showed its ability to detect a scant amount of ascitic fluid that US and CT could not detect in 4 patients with pancreatic ductal adenocarcinoma. In these patients, the average amount of aspirated fluid was only 2.6 mL. Two of them were diagnosed as malignant, and this result changed their management (Figure 1 A-C)24).
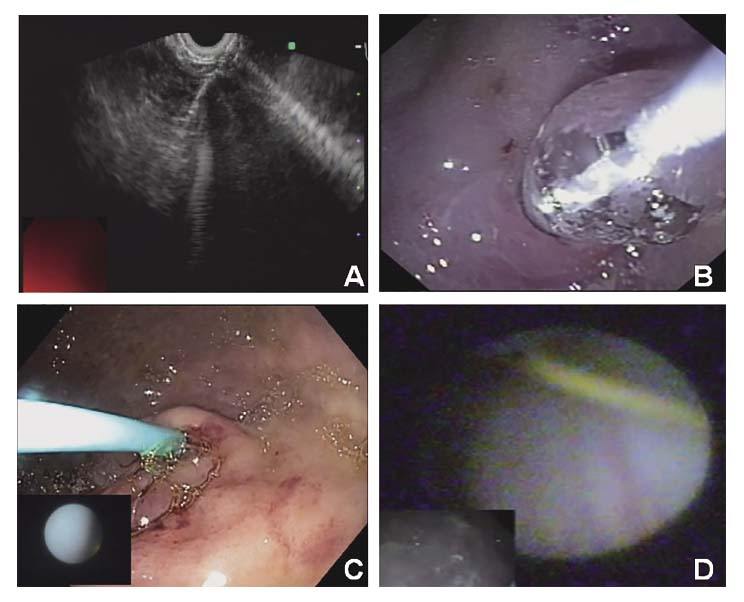
Another issue in staging is preoperative laparoscopy, which has been used in the staging of various types of cancers. Even though it has been proven to be more accurate for decisions of resectability than other cross-sectional imaging, a lack of validated studies and its complexity for this purpose (e.g., need for general anesthesia and an operating room) hinder its application in routine practice. To resolve this issue, we conducted an animal study that aimed to develop a new endoscopic procedure for exploration of the abdominal cavity. Our pilot study demonstrated the technical feasibility of EUS-assisted direct peritoneal visualization with a 10-Fr small-caliber scope in a swine model. As we hypothesized, a <1-cm stomach wall defect can be closed tightly with commercially available hemostasis clips without any special techniques (Figure 2 A-D)25).
2. Para-aortic lymph node metastasis
Para-aortic lymph node (PALN) metastasis is classified as a distant metastasis and is regarded as one of the unresectable factors. Recent reports demonstrated that such PALN metastases were found in more than 10% of surgical cases of PC. However, because of the limited diagnostic ability using conventional imaging modalities (eg, ultrasonography, multidetector-row CT [MDCT], magnetic resonance imaging, EUS, and positron emission tomography [PET]), PALN metastasis currently is not always evaluated before surgery. Kurita et al. conducted a prospective study to compare the diagnostic yield for PALN metastases between EUS-FNA and PET-CT26). In their analysis, they found that preoperative EUS-FNA or PET/CT made a correct diagnosis in 20 (95.2%) and 12 (57.1%), respectively. EUS-FNA had higher sensitivity and specificity for the diagnosis of PALN metastases (sensitivity, 96.7% [29/30];95% confidence interval, 82.2%-99.9%;specificity, 100% [39/39]; 95% confidence interval, 91.0%-100%) than PET/CT. Considering this result, they concluded that EUS-FNA should be part of the standard preoperative examination for patients with PC.
EUS-FNA in precision medicine for pancreatic cancer
Precision medicine involves providing specific treatments for patients who have tumors with certain types of bio-profiles. In the last three decades, precision medicine has developed dramatically and is now applied to many different cancers, including PC. Techniques for precision medicine range from ex vivo cancer models, immunohistochemistry (IHC), real time-PCR, and mutational analysis using next-generation sequencing (NGS).
Our groups previously reported the utility of chemo-sensitivity testing by using EUS-FNA samples in patients with unresectable PC27). This study included 34 patients, and chemo-sensitivity (treated/control ratio:T/C ratio) was calculated as the quantity of adenosine triphosphate for a tumor treated with gemcitabine as a percentage of that for the control. When the cut-off value of the T/C ratio was set at 74%, we could predict 180-day progression-free survival (PFS) with sensitivity and specificity of 71.4% and 91.3%, respectively.
Additionally, analyses of drug sensitivity-related gene profiles using samples obtained by EUS-FNA have been reported. Ashida et al. extracted messenger RNA from EUS-FNA samples and conducted cDNA array analysis28). They found that dCK mRNA expression is a candidate indicator for GEM efficacy in unresectable PC. Quantitative mRNA measurements of deoxycytidine kinase using EUS-FNA samples are necessary for definitive conclusions. With a similar method, Eto et al. found that human equilibrative nucleoside transporter 1 and Notch3 mRNA expressions in EUS-FNA specimens were the key predictive biomarkers of GEM effect and GEM sensitivity in patients with unresectable PC29).
Using NGS, an entire human genome can be sequenced within a single day. First, several studies have used this technique for the diagnosis of PC30,31). Subsequently, in the era of precision medicine, its application has shifted from diagnosis to molecular profiling for potential targets for personalized anticancer therapy. Even though we still have many issues that limit the clinical application of precision medicine to PC, EUS-FNA may play an important role in precision medicine for PC in the future32).
Conflict of Interest Disclosure
The authors declare no conflicts of interest associated with this manuscript.
References
- 1. Wiersema MJ, Vilmann P, Giovannini M, et al. Endosonography-guided fine-needle aspiration biopsy: diagnostic accuracy and complication assessment. Gastroenterology, 112: 1087-1095, 1997.
- 2. Siegel R, Ma J, Zou Z, et al. Cancer statistics, 2014. CA Cancer J Clin, 64 9-29, 2014.
- 3. Gillen S, Schuster T, Meyer Zum Buschenfelde C, et al. Preoperative/neoadjuvant therapy in pancreatic cancer: a systematic review and meta-analysis of response and resection percentages. PLoS Med, 7: e1000267, 2010.
- 4. Kamisawa T, Wood LD, Itoi T, et al. Pancreatic cancer. Lancet, 388: 73-85, 2016.
- 5. Wakatsuki T, Irisawa A, Bhutani MS, et al. Comparative study of diagnostic value of cytologic sampling by endoscopic ultrasonography-guided fine-needle aspiration and that by endoscopic retrograde pancreatography for the management of pancreatic mass without biliary stricture. J Gastroenterol Hepatol, 20: 1707-1711, 2005.
- 6. Committee ASoP, Eloubeidi MA, Decker GA, et al. The role of endoscopy in the evaluation and management of patients with solid pancreatic neoplasia. Gastrointest Endosc, 83: 17-28, 2016.
- 7. Hewitt MJ, McPhail MJ, Possamai L, et al. EUS-guided FNA for diagnosis of solid pancreatic neoplasms: a meta-analysis. Gastrointest Endosc, 75: 319-331, 2012.
- 8. Eloubeidi MA, Tamhane A, Jhala N, et al. Agreement between rapid onsite and final cytologic interpretations of EUS-guided FNA specimens: implications for the endosonographer and patient management. Am J Gastroenterol, 101: 2841-2847, 2006.
- 9. Schmidt RL, Witt BL, Lopez-Calderon LE, et al. The influence of rapid onsite evaluation on the adequacy rate of fine-needle aspiration cytology: a systematic review and meta-analysis. Am J Clin Pathol, 139: 300-308, 2013.
- 10. Hikichi T, Irisawa A, Bhutani MS, et al. Endoscopic ultrasound-guided fine-needle aspiration of solid pancreatic masses with rapid on-site cytological evaluation by endosonographers without attendance of cytopathologists. J Gastroenterol, 44: 322-328, 2009.
- 11. Suzuki R, Irisawa A, Bhutani MS, et al. Prospective evaluation of the optimal number of 25-gauge needle passes for endoscopic ultrasound-guided fine-needle aspiration biopsy of solid pancreatic lesions in the absence of an onsite cytopathologist. Dig Endosc, 24: 452-456, 2012.
- 12. Ge PS, Wani S, Watson RR, et al. Per-pass performance characteristics of endoscopic ultrasound-guided fine-needle aspiration of malignant solid pancreatic masses in a large multicenter cohort. Pancreas, 47: 296-301, 2018.
- 13. Uehara H, Sueyoshi H, Takada R, et al. Optimal number of needle passes in endoscopic ultrasound-guided fine needle aspiration for pancreatic lesions. Pancreatology, 15: 392-396, 2015.
- 14. Rosch T, Braig C, Gain T, et al. Staging of pancreatic and ampullary carcinoma by endoscopic ultrasonography. Comparison with conventional sonography, computed tomography, and angiography. Gastroenterology, 102: 188-199, 1992.
- 15. Palazzo L, Roseau G, Gayet B, et al. Endoscopic ultrasonography in the diagnosis and staging of pancreatic adenocarcinoma. Results of a prospective study with comparison to ultrasonography and CT scan. Endoscopy, 25: 143-150, 1993.
- 16. Muller MF, Meyenberger C, Bertschinger P, et al. Pancreatic tumors: evaluation with endoscopic US, CT, and MR imaging. Radiology, 190: 745-751, 1994.
- 17. Gress FG, Hawes RH, Savides TJ, et al. Role of EUS in the preoperative staging of pancreatic cancer: a large single-center experience. Gastrointest Endosc, 50: 786-791, 1999.
- 18. Ahmad NA, Lewis JD, Siegelman ES, et al. Role of endoscopic ultrasound and magnetic resonance imaging in the preoperative staging of pancreatic adenocarcinoma. Am J Gastroenterol, 95: 1926-1931, 2000.
- 19. Midwinter MJ, Beveridge CJ, Wilsdon JB, et al. Correlation between spiral computed tomography, endoscopic ultrasonography and findings at operation in pancreatic and ampullary tumours. Br J Surg, 86: 189-193, 1999.
- 20. Shami VM, Mahajan A, Loch MM, et al. Comparison between endoscopic ultrasound and magnetic resonance imaging for the staging of pancreatic cancer. Pancreas, 40: 567-570, 2011.
- 21. DeWitt J, LeBlanc J, McHenry L, et al. Endoscopic ultrasound-guided fine-needle aspiration of ascites. Clin Gastroenterol Hepatol, 5: 609-615, 2007.
- 22. Kaushik N, Khalid A, Brody D, et al. EUS-guided paracentesis for the diagnosis of malignant ascites. Gastrointest Endosc, 64: 908-913, 2006.
- 23. Nguyen PT, Chang KJ. EUS in the detection of ascites and EUS-guided paracentesis. Gastrointest Endosc, 54: 336-339, 2001.
- 24. Suzuki R, Irisawa A, Bhutani MS, et al. An automated spring-loaded needle for endoscopic ultrasound-guided abdominal paracentesis in cancer patients. World J Gastrointest Endosc, 6: 55-59, 2014.
- 25. Suzuki R, Bhutani MS, Shin D, et al. Endoscopic ultrasound-assisted direct peritoneal visualization with a small-caliber scope: A proof of concept study in a swine model. Endosc Ultrasound, 3: 226-231, 2014.
- 26. Kurita A, Kodama Y, Nakamoto Y, et al. Impact of EUS-FNA for preoperative para-aortic lymph node staging in patients with pancreatobiliary cancer. Gastrointest Endosc, 84: 467-475 e461, 2016.
- 27. Wakatsuki T, Irisawa A, Terashima M, et al. ATP assay-guided chemosensitivity testing for gemcitabine with biopsy specimens obtained from unresectable pancreatic cancer using endoscopic ultrasonography-guided fine-needle aspiration. Int J Clin Oncol, 16: 387-394, 2011.
- 28. Ashida R, Nakata B, Shigekawa M, et al. Gemcitabine sensitivity-related mRNA expression in endoscopic ultrasound-guided fine-needle aspiration biopsy of unresectable pancreatic cancer. J Exp Clin Cancer Res, 28: 83, 2009.
- 29. Eto K, Kawakami H, Kuwatani M, et al. Human equilibrative nucleoside transporter 1 and Notch3 can predict gemcitabine effects in patients with unresectable pancreatic cancer. Br J Cancer, 108: 1488-1494, 2013.
- 30. Kameta E, Sugimori K, Kaneko T, et al. Diagnosis of pancreatic lesions collected by endoscopic ultrasound-guided fine-needle aspiration using next-generation sequencing. Oncol Lett, 12: 3875-3881, 2016.
- 31. de Biase D, Visani M, Baccarini P, et al. Next generation sequencing improves the accuracy of KRAS mutation analysis in endoscopic ultrasound fine needle aspiration pancreatic lesions. PloS One, 9: e87651, 2014.
- 32. Berry W, Lundy J, Croagh D, et al. Reviewing the utility of EUS FNA to advance precision medicine in pancreatic cancer. Cancers (Basel), 10: 2018.