2022 年 68 巻 2 号 p. 129-134
2022 年 68 巻 2 号 p. 129-134
A 768 g female neonate, born at 25 weeks’ gestation, developed sepsis due to methicillin-resistant Staphylococcus epidermidis on day 14. Severe thrombocytopenia was observed, and hemophagocytic macrophages were identified in her peripheral blood smear. Cytokine profiles at the time of onset suggested that an inflammatory cytokine storm had activated lymphocytes and macrophages, leading to platelet phagocytosis. After administration of vancomycin for 14 days and immunoglobulin therapy, she improved without any complications. Considering the results of cytokine profiles, early intervention for infection may have prevented progression to hemophagocytic lymphohistiocytosis and reduced the severity of clinical symptoms.
Thrombocytopenia is one of the most frequent hematologic abnormalities in the neonatal period, with sepsis being among the most common causes of severe neonatal thrombocytopenia. We encountered a case of an extremely low birth weight infant who developed sepsis on day 14 and whose peripheral blood smear showed many images of platelet phagocytosis by macrophages. To the best of our knowledge, this is the first reported case of neonatal sepsis with hemophagocytic macrophages identified in a peripheral blood smear. We report the pathophysiology from the perspective of the infant’s cytokine profiles at the time of onset.
A 768 g female was delivered by emergency cesarean section at 25 weeks’ gestation, due to tocolysis failure. The baby was intubated immediately and admitted to our neonatal intensive care unit (NICU). Chest radiographic findings were compatible with respiratory distress syndrome, and artificial lung surfactant (Surfacten®, Mitsubishi Tanabe Pharma, Osaka, Japan) was administered. High-frequency oscillatory ventilation mode was selected as the initial ventilator setting. Empirical antibiotics (ampicillin and gentamicin) were administered for 3 days.
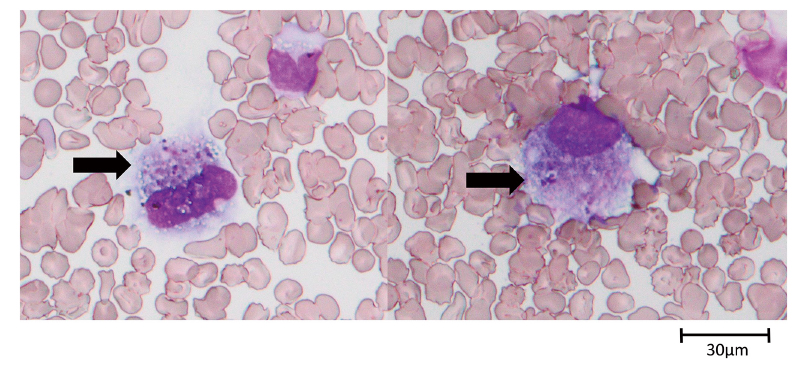
On day 14, the patient’s condition worsened. Although the anterior fontanelle was flat and there were no abnormal cardiopulmonary findings, there was decreased activity and poor skin color. Blood examination revealed an increased inflammatory response and severe thrombocytopenia (Table 1). No coagulopathy was observed, nor did test results meet the diagnostic criteria for neonatal disseminated intravascular coagulation. However, many images of platelet phagocytosis by macrophages were observed in her peripheral blood smear (Figure 1). The patient was diagnosed as having clinical sepsis. Because Enterobacter and coagulase-negative staphylococcus were detected in nasal culture in the most recent result of once-a-week surveillance, these were assumed to be the causative organisms. She was thus started on intravenous administration of antibiotics (amikacin 8 mg/kg/day and vancomycin 20 mg/kg/day) and immunoglobulin therapy (200 mg/kg/day for 3 days). Platelet transfusion was also performed for 2 days.
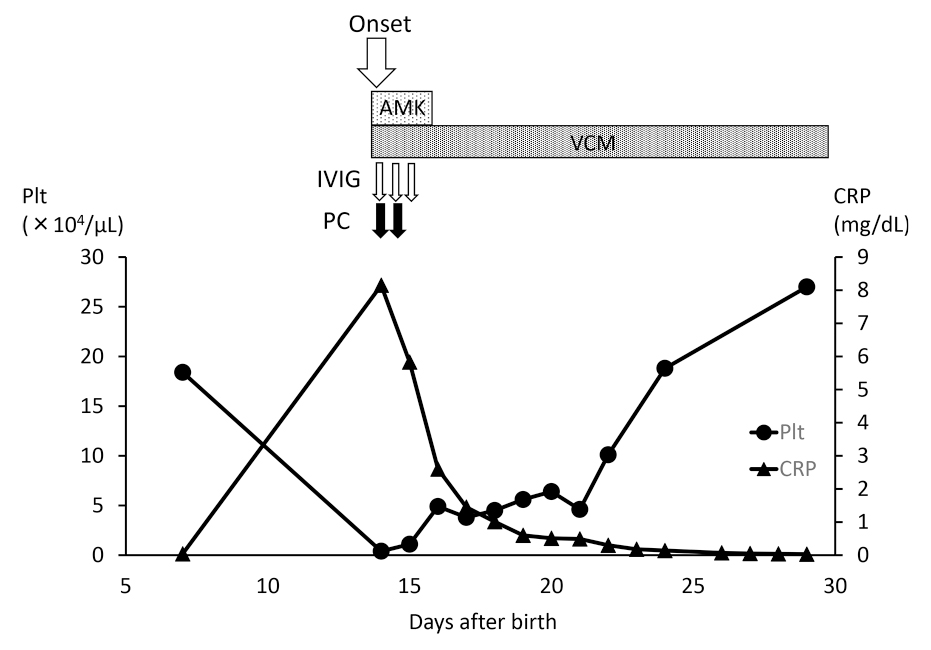
On day 15, the blood culture was positive and the causative agent was found to be methicillin-resistant Staphylococcus epidermidis (MRSE), so antibiotic therapy was continued only with vancomycin, to which the organism was sensitive. After the start of medication, her C-reactive protein decreased steadily, and her platelet count also improved gradually (Figure 2). Vancomycin was administered for 14 days, after which the infection did not recur.
On day 46, the baby was extubated and the ventilation mode was switched to nasal continuous positive airway pressure that continued for a further 27 days. She was discharged on day 110 without sequelae.
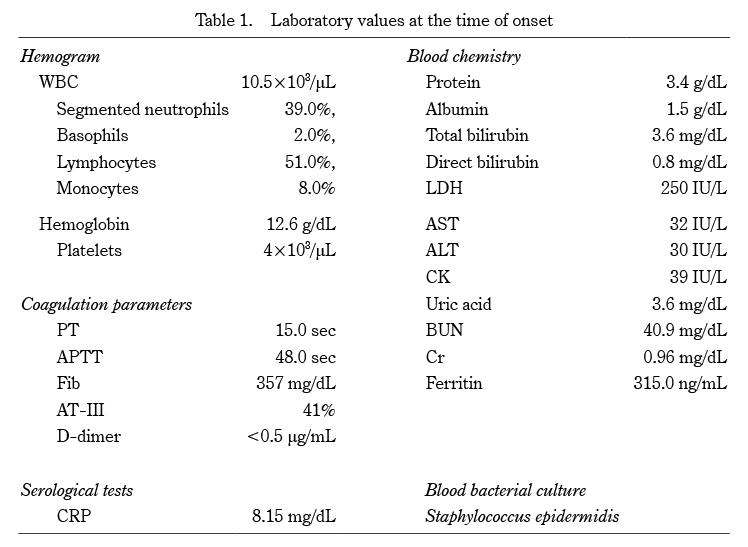
WBC:white blood cell, PT:prothrombin time, APTT:activated partial thromboplastin time, Fib:fibrinogen, AT-III:antithrombin-III, CRP:C-reactive protein, LDH:lactate dehydrogenase, AST:aspartate aminotransferase, ALT:alanine aminotransferase, CK:creatine kinase, BUN:blood urea nitrogen, Cr:creatinine
We measured serum cytokine levels with the BioPlex protein array system (Bio-Rad, Alameda, CA, USA), as described previously1), using the BioPlex human cytokine 17-plex panel.
The results of cytokine profiles at onset and 12 hours after onset are shown in Table 2. The cytokine data at onset are before IVIG administration and data at 12 hours after onset are after IVIG administration. Abnormally high values were observed in 11 items, including tumor necrosis factor-α (TNF-α), interleukin (IL)-6, interferon (IFN)-γ, IL-2, IL-5, IL-10, IL-17, granulocyte colony stimulating factor, IL-8, monocyte chemoattractant protein-1 (MCP-1), and macrophage inflammatory protein-1β (MIP-1β). Among these, levels of TNF-α, IL-6, IFN-γ, IL-10, IL-8, and MCP-1 were strikingly high, whereas the level of granulocyte/macrophage colony stimulating factor was not high. Reference control values were obtained from Takahashi et al1).

CB:cord blood, SD:standard deviation, OOR:out of range, TNF:tumor necrosis factor, IL:interleukin, IFN:interferon, GM-CSF:granulocyte/macrophage colony stimulating factor, G-CSF:granulocyte colony stimulating factor, MCP-1:monocyte chemoattractant protein-1, MIP-1β:macrophage inflammatory protein-1β.
We obtained informed consent from the patient’s parents and this study was approved by the ethics committee of the Japanese Red Cross Musashino Hospital (3090).
Thrombocytopenia is one of the most frequent hematologic abnormalities occurring in the neonatal period. It affects about 18-35% of all patients admitted to the NICU, with sepsis being among the most common cause of severe neonatal thrombocytopenia2,3). Thrombocytopenia is one of the most predictive, independent risk factors for sepsis-associated mortality in very low birth weight infants4). It may be due to suppression of hematopoiesis or increased consumption and destruction.However, the mechanism of thrombocytopenia in neonatal sepsis has not been clearly elucidated.
In the present case, many images of platelet phagocytosis by macrophages were observed in her peripheral blood smear, and increased destruction may have been the cause of her thrombocytopenia. The fact that platelets increased steadily after only two platelet transfusions likely proved that the cause of thrombocytopenia in this infant was not the suppression of platelet production.
Cytological evidence of hemophagocytosis is usually found in bone marrow films or in biopsies of other organs such as lymph nodes, liver, spleen, and skin5). The observation of hemophagocytic macrophages in a peripheral blood smear is uncommon. Kaga et al.6) reported a neonate with neonatal toxic shock syndrome (TSS)-like exanthematous disease (NTED) complicated by hemophagocytic syndrome (HPS). In this case, the patient did not meet the diagnostic criteria of HPS, so-called hemophagocytic lymphohistiocytosis (HLH).7) Compared with the cytokine profile measurement groups we have reported to date and HLH group (Table 3), this infant’s TNF-α was strikingly high, as was her IL-6 and IFN-γ. In addition, the values of three chemokines, IL-8, MCP-1, and MIP-1β, were also high. As we reported previously, even extremely low birth weight infants can suffer from cytokine storms12). Considering the cytokine profile results, we speculated that a cytokine storm occurred with activation of lymphocytes and excessive and inappropriate activation of macrophages, which phagocytose platelets. We could find no further phagocytosis of macrophages in the peripheral blood smear 12 hours after the onset of the disease. Comparing the cytokine profiles at the two times, a significant decrease in inflammatory cytokines and chemokines occurred over a short period of time (Table 2), which may have led to a short stimulation of hemophagocytosis and a good recovery of the infant’s platelet count.
In adults, detection of peripheral hemophagocytosis is thought to reflect lethal clinical conditions13). In the present infant, although thrombocytopenia was severe, her clinical course was not serious. In a report of pediatric HLH, IFN-γ and IL-10 were highly elevated, and IL-6 was moderately elevated11). While our infant’s cytokine profiles were close to those of HLH, we speculate that she did not progress to HLH because inflammatory cytokines were lowered at an early stage due to early intervention for infection. The fact that inflammatory cytokines and chemokines were markedly reduced in a short period of time, as noted above, also supports this hypothesis.
In conclusion, this may be the first report of an extremely low birth weight infant with severe thrombocytopenia during sepsis and platelet phagocytosis on peripheral blood smear. When severe thrombocytopenia is observed in neonatal sepsis, it is important to check the peripheral blood smear carefully; measurement of the cytokine profile may help clarify the pathogenesis of the disease.
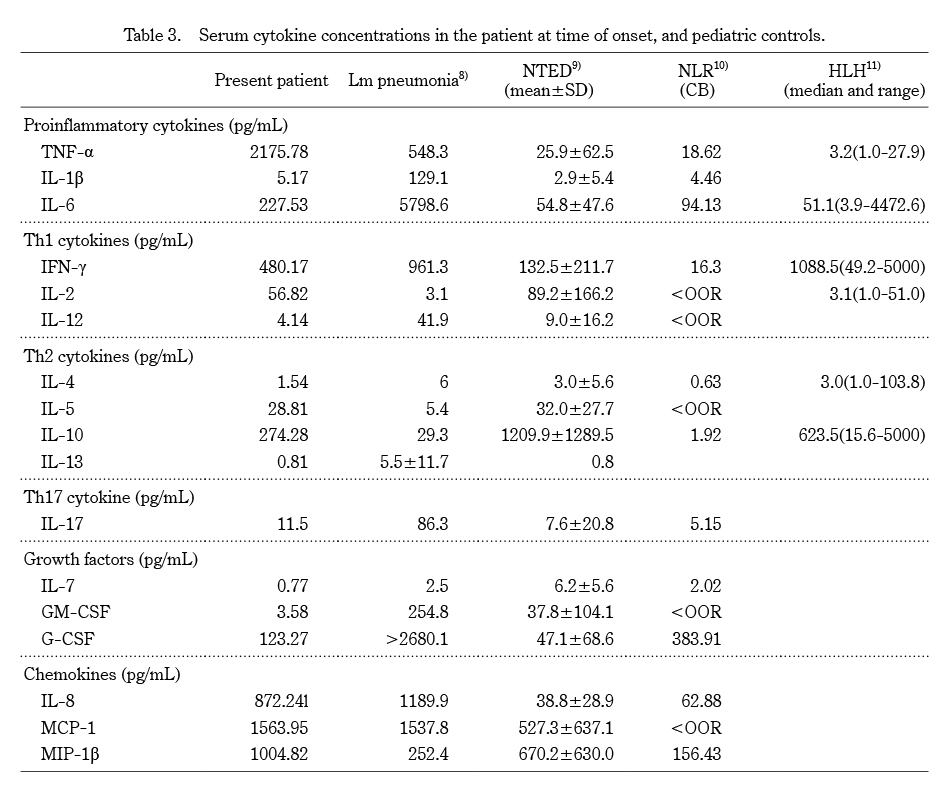
Lm:Listeria monocytogenes, NTED:neonatal toxic shock-like exanthematous disease, NLR:neonatal leukemoid reaction, CB:cord blood, HLH:hemophagocytic lymphohistiocytosis, SD:standard deviation, OOR:out of range, TNF:tumor necrosis factor, IL:interleukin, IFN:interferon, GM-CSF:granulocyte/macrophage colony stimulating factor, G-CSF:granulocyte colony stimulating factor, MCP-1:monocyte chemoattractant protein-1, MIP-1β:macrophage inflammatory protein-1β
The authors declare no conflicts of interest.
D.H. wrote the manuscript. N.T. performed the cytokine measurements. K.I., E.F., T.N., M.K., H.T., T.N., and N.T. gave conceptual advice. All authors read and approved the final manuscript.