ABSTRACT
Aim: Peppermint oil, which suppresses gastric peristalsis during esophagogastroduodenoscopy (EGD), is effective for determining the margin of a gastric tumor. This study was conducted to evaluate the utility of an L-menthol preparation for suppressing gastric peristalsis and for diagnosing gastric tumors.
Methods: The study examined 124 patients who underwent EGD between January and April 2012. After 20 mL of 0.8% L-menthol was sprayed directly onto the mucosal surface of the gastric antrum, the degree of peristalsis suppression in the antrum was evaluated. The effectiveness of L-menthol for identifying new gastric tumors and determining tumor margins was also evaluated.
Results: Gastric peristalsis was suppressed in 88.5% (69/78) of patients, with complete suppression of peristalsis achieved in 78.2%. L-menthol exerted a higher peristalsis-suppressive effect in patients with endoscopic gastric mucosal atrophy (93.3%, 56/60) than in patients without atrophy (72.2%, 13/18) (p = .014). L-menthol application caused the detection of new gastric tumors in 1.6% (2/124) of patients and clarification of the margin of three lesions (in 3 patients) identified as having an unclear margin before L-menthol application.
Conclusion: These results suggest that L-menthol is effective for suppressing gastric peristalsis during EGD and suggest that it is useful for identifying gastric tumors and for determining tumor margins.
INTRODUCTION
Antispasmodics such as butylscopolamine bromide and glucagon have been used to suppress gastrointestinal peristalsis during esophagogastroduodenoscopy (EGD). However, the use of antispasmodics is restricted in patients with comorbidities such as cardiac disease, glaucoma, prostatic hypertrophy, and diabetes, leading to an increasing number of patients contraindicated for these drugs in an aging society. The inability to use antispasmodics might increase the risk of overlooked gastric tumors because of vigorous peristalsis. Moreover, conventional antispasmodics are administered by intramuscular injection, exposing healthcare professionals to the risk of needle-stick injury and exposing patients to the burden of pain.
Since the efficacy of peppermint oil-containing enteric capsules in treating irritable bowel syndrome was reported by Rees et al. in 19791), peppermint oil has also been shown to be effective as an antispasmodic for use during lower gastrointestinal endoscopy2) and barium enema examination3). In 2003, a Japanese group led by Hiki et al.4) compared the efficacy and safety of 1.6% peppermint oil suspension administered through a forceps channel of an endoscope into the stomach during EGD with those of butylscopolamine bromide administered intramuscularly in a double-blind study. They demonstrated that peppermint oil suspension was more effective in suppressing the contraction motion of the pyloric ring and caused fewer adverse reactions. Based on these findings reported by Hiki et al., we started in-hospital prescription of 1.6% peppermint oil from 2008. This report describes its efficacy for peristalsis suppression and its high acceptance by patients5), as well as its usefulness in identifying new gastric tumors and determining tumor margins6). However, several problems arise when using internally prescribed peppermint oil, such as inconsistency in the contents of the main ingredient L-menthol and phase separation into aqueous and oil phases occurring over time after preparation. An L-menthol preparation that overcame these shortcomings was launched in Japan in January 2011. It has been made available for use during EGD. This product contains L-menthol as the active ingredient at a concentration of 0.8%, which is lower than that of our internally prescribed peppermint oil. This study was conducted to verify the usefulness of the L-menthol preparation as an antispasmodic for EGD, as reported previously by Hiki et al.,7) in addition to its effectiveness for detecting and diagnosing gastric tumors.
METHODS
Patients
This study examined 124 consecutive patients who underwent EGD between January and April 2012 performed by an endoscopist (T.H.), a board-certified instructor of the Japan Gastroenterological Endoscopy Society, who met the inclusion criteria presented below. Data for these patients were analyzed retrospectively between March and July 2014. To be included, patients had to be at least 20 years old, without known hypersensitivity to L-menthol. Patients were excluded from the study if they had a previous history of gastric surgery, any disease that might cause reduced gastric peristalsis (e.g. diabetes), or if they had known hypersensitivity to L-menthol. This study was conducted based on Fukushima Medical University Ethics Committee Approval No. 1954.
Endoscopic procedure
Each patient was instructed to drink a mixture of 20,000 units of pronase (Pronase MS®; Kaken Pharmaceutical Co. Ltd., Tokyo, Japan), 80 mg dimethicone (Balgin Antifoaming Oral Solution 2%®; Kaigen Pharma Co. Ltd., Osaka, Japan), and 1 g sodium bicarbonate in 80 mL of water 10 min before scope insertion. Immediately before insertion of the endoscope (GIF-H260; Olympus Medical Systems Corp., Tokyo, Japan), 40 mg lidocaine (Xylocaine Pump Spray 8%; AstraZeneca K.K., Osaka, Japan) was sprayed into the oral cavity for pharyngeal anesthesia8).
Gastric peristalsis suppressing effect
The endoscope was inserted into the gastric antrum, at which gastric peristalsis was observed for 20 s. Gastric peristalsis was evaluated based on the Niwa classification9) into Grade 1 (no peristalsis), Grade 2 (peristalsis not involving the pyloric ring), or Grade 3 (peristalsis involving the pyloric ring). After peristalsis evaluation, 20 mL of 0.8% L-menthol (Mincrea®; Nihon Pharmaceutical Co. Ltd., Tokyo, Japan) was sprayed directly through a forceps channel of the scope onto the gastric antrum. Then, peristalsis was observed again for 20 s in the antrum to evaluate the peristalsis-suppressive effect of L-menthol in the antrum. The degree of peristalsis suppression was defined as “complete suppression” if peristalsis was stopped after L-menthol application, “mild suppression” if peristalsis was not suppressed completely but reduced compared to the pre-application state, or “no suppression” if no difference in peristalsis was observed between states before and after application. For patients with a pre-application peristalsis Grade of 2 or 3, the degree of peristalsis suppression was evaluated according to the presence or absence of endoscopic gastric mucosal atrophy. After EGD, each patient was instructed to rest for 1 hr in the examination room, where they were monitored for adverse events.
Effectiveness in diagnosing gastric tumors
The L-menthol effectiveness was evaluated for the identification of new gastric tumors in the gastric antrum that were not recognized before EGD or identified before L-menthol application. The effectiveness was also evaluated for clarifying the margins of gastric tumors that were identified before EGD with an unclear margin.
Statistical evaluation
For analysis of patient characteristics, the patient age was expressed as mean ± standard deviation. The degrees of peristalsis suppression in patients with and without endoscopic gastric mucosal atrophy were analyzed using chi-square tests, with significant difference inferred for p < .05. All statistical analyses were conducted using software (Excel Statistics; OMS Publishing Inc., Saitama, Japan).
RESULTS
Patient characteristics
Table 1 presents characteristics of the 79 male and 45 female patients. Their mean age was 66.7 ± 12.9 years. With regard to underlying disease, half of the patients (62/124) had treated or untreated gastric or esophageal cancer. Regarding background gastric mucosal findings, 78.2% (97/124) of the patients were diagnosed endoscopically as having atrophic gastritis.
Gastric peristalsis suppressing effect
In the evaluation of gastric peristalsis before L-menthol application, 37.1% (46/124) of the patients were classified as Grade 1, 13.7% (17/124) as Grade 2 and 49.2% (61/124) as Grade 3 (Fig. 1). Of 78 patients classified as Grade 2 or 3, 69 (88.5%) patients had their gastric peristalsis suppressed by L-menthol, with complete suppression achieved in 78.2% (61/78) and mild suppression in 10.3% (8/78) (Fig. 2).
The 78 patients classified as having Grade 2 or 3 peristalsis before L-menthol application were divided into 60 (76.9%) patients with and 18 (23.1%) patients without endoscopic gastric mucosal atrophy. In those with mucosal atrophy, peristalsis was suppressed completely in 80.0% (48/60), mildly suppressed in 13.3% (8/60), and not suppressed in 6.7% (4/60). The corresponding percentages in those without mucosal atrophy were 72.2% (13/18), 0% (0/18), and 27.8% (5/18) (Fig. 3). This result presents a higher peristalsis-suppressive effect exerted in those with gastric mucosal atrophy than in those without (p = .014).
Adverse events included nausea and a generally bad feeling experienced by one patient (0.8%) after EGD.
Effectiveness in diagnosing gastric tumors
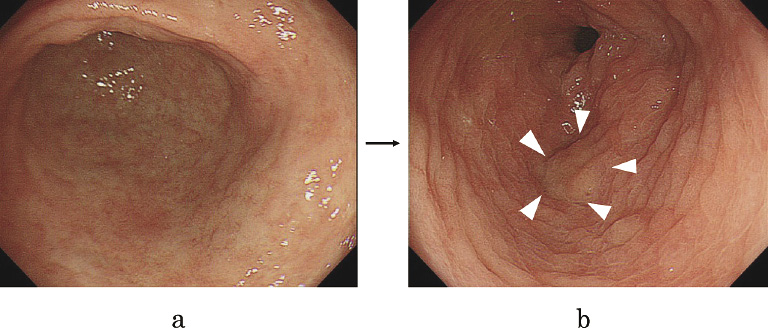
L-menthol application led to the detection of new gastric tumors in 1.6% (2/124) of the patients in whom no tumor had been identified in the gastric antrum before EGD. The 20-s endoscopic observation performed before L-menthol application failed to detect any tumor. In both cases, tumors were detected before the scope was advanced into the duodenum. One tumor, found in the lesser curvature of the antrum as a depressed type cancer (0-IIc), was diagnosed as well differentiated adenocarcinoma (tub1) based on results of biopsy (Fig. 4). The tub1 lesion was treated later by endoscopic submucosal dissection (ESD) (Fig. 5). The other tumor, found in the posterior wall of the antrum as a raised lesion, was diagnosed as an adenoma based on results of biopsy (Fig. 6).
In terms of its effectiveness in determining tumor margins, L-menthol sprayed directly on gastric tumors clarified their margins in three patients in whom lesions were identified with an unclear margin before L-menthol application, including one patient with type 0-IIc cancer in the gastric antrum (Fig. 7) and two patients with type 0-IIc cancer in the gastric corpus. L-menthol application caused an edematous change of the non-tumorous gastric mucosa, which clarified the tumor margin.
DISCUSSION
During endoscopy, gastric peristalsis is observed mainly in the antrum. In the presence of vigorous peristalsis, small gastric carcinomas and ulcers can be masked and overlooked. The difficulty in advancing the scope through the pyloric ring into the duodenum engenders a prolonged operation time. Therefore, the absence of gastric peristalsis is a pre-requisite for better diagnostic accuracy and reduced pain experienced by a patient during EGD. As a substitution for the conventional antispasmodics, such as butylscopolamine bromide and glucagon, that are administered by intramuscular injection, an L-menthol preparation has been developed recently for direct application to gastric mucosa through a forceps channel of an endoscope.
L-menthol has been shown to inhibit contraction of gastrointestinal smooth muscles in experiments using guinea pig ileum10,11), rabbit jejunum11,12), and the human colon13). L-menthol has also been shown to suppress spontaneous contraction of rabbit jejunum smooth muscle dose-dependently and to exert an immediate and sustained suppressive effect on gastric peristalsis in dogs and monkeys12). Actually, L-menthol is believed to bind to L-type voltage-dependent calcium channels present on the cell membrane of gastrointestinal smooth muscle cells. Thereby, it blocks the influx of calcium ions into cells, resulting in the loss of membrane potential generation and the subsequent relaxation of smooth muscles10, 11).
In this study, L-menthol application caused suppressed gastric peristalsis in 88.5% of patients in whom peristalsis was observed before L-menthol application. Moreover, complete suppression of peristalsis was achieved in 78.2% of patients, demonstrating a strong gastric peristalsis-suppressive effect of the L-menthol preparation. This effect was particularly pronounced in patients with gastric mucosal atrophy compared to those without atrophy. A possible explanation for this difference is that, although we conducted no histological evaluation of the atrophied region or intestinal metaplasia, in the presence of gastric mucosal atrophy, progression from atrophic gastritis to intestinal metaplasia increases the number of absorptive cells at the affected site, thereby resulting in increased absorption of L-menthol and subsequent enhancement of its peristalsis-suppressive effect. Although Hiki et al. 7) reported that the rate of complete suppression of gastric peristalsis was 37.5%, it was 78.2% in the present study. This difference is regarded as attributable to the differences in the evaluation time of the gastric peristalsis-suppressive effect. Hiki et al. evaluated the gastric peristalsis-suppressive effect twice immediately after L-menthol application and at the end of EGD. Each of the evaluation times was 45 s. In contrast, it was 20 s after L-menthol application in this study. In fact, the peristalsis of the stomach is a problem during observation only from the antrum to pylorus. Therefore, 20 s of observation time is sufficient to determine gastric peristalsis suppression, although 45 s was regarded as painful for the patients.
New tumors were detected in the gastric antrum in two patients in whom no tumor had been identified before L-menthol application, probably because the application of the L-menthol preparation to gastric mucosa affected by atrophic gastritis or intestinal metaplasia caused edematous change of the background mucosa around the tumor, making the surface irregularity more prominent and thereby making tumor detection easier. It is also likely that the same mechanism underlies the additional effectiveness of L-menthol in determining the tumor margin. Mori et al.14) reported that L-menthol application causes an edematous change of gastric mucosa and thereby clarifies the margin of gastric lesions, such as erosion, ulcer, and early stage cancer, which we have also demonstrated in a study using peppermint oil6).
The results reported herein demonstrate that the L-menthol preparation is useful as a safe and convenient suppressor of gastric peristalsis and also that it is useful for identifying new gastric tumors and determining the margin of gastric tumors. This study includes some limitations. First, the study was conducted at a single institution by a single operator. It included only a few patients. The additional efficacy of L-menthol in tumor diagnosis was based on a subjective evaluation by a single operator. The evaluation of background gastric mucosa was done only by endoscopy. No histological evaluation was performed. No examination of Helicobacter pylori infection was performed in most cases.
Additional studies are being planned for histologic examination of the changes in gastric mucosa caused by L-menthol application and for an objective demonstration of differential image contrast between a gastric tumor and surrounding non-tumorous mucosa.
ACKNOWLEDGEMENT
We express our gratitude to all endoscopy medical staff for their collaboration and assistance with endoscopic procedures.
CONFLICT OF INTEREST
The authors have no conflict of interest in relation to this study.
REFERENCES
- 1. Rees WD, Evans BK, Rhodes J. Treating irritable bowel syndrome with peppermint oil. Br Med J, 6: 835-836, 1979.
- 2. Leicester RJ, Hunt RH. Peppermint oil to reduce colonic spasm during endoscopy. Lancet, 2: 989, 1982.
- 3. Sparks MJ, O’Sullivan P, Herrington AA, Morcos SK. Does peppermint oil relieve spasm during barium enema? Br J Radiol, 68: 841-843, 1995.
- 4. Hiki N, Kurosaka H, Tatsutomi Y, et al. Peppermint oil reduces gastric spasm during upper endoscopy: a randomized, double-blind, double-dummy controlled trial. Gastrointest Endosc, 57: 475-482, 2003.
- 5. Mizuno Y, Hikichi T, Suzuki T, et al. Peppermint oil is useful as an antispasmodic agent in esophago-gastro-duodenoscopy. Fukushima Med J, 57: 9-16, 2007. (in Japanese with English abstract)
- 6. Hikichi T, Irisawa A, Sato M, et al. Utility of peppermint oil for endoscopic diagnosis of gastric tumors. Fukushima J Med Sci, 57: 60-65, 2011.
- 7. Hiki N, Kaminishi M, Tanabe S, et al. An open-label, single-arm study assessing the efficacy and safety of L-menthol sprayed onto the gastric mucosa during upper gastrointestinal endoscopy. J Gastroenterol, 46: 873-882, 2001.
- 8. Mizuno Y, Hikichi T, Itabashi M, et al. A comparative study of the effect and discomfort produced by pharyngeal anesthesia with viscous lidocaine solution and with lidocaine spray in esophagogastroduodenoscopy. Fukushima Med J, 61: 12-17, 2010. (in Japanese with English abstract)
- 9. Niwa H, Nakamura T, Fujino M. Endoscopic observation on gastric peristalsis and pyloric movement. Gastroenterol Endosc, 17: 236-242, 1975. (in Japanese with English abstract)
- 10. Hawthorn M, Ferrante J, Luchowski E, Rutledge A, Wei XY, Triggle DJ. The actions of peppermint oil and menthol on calcium channel dependent processes in intestinal, neuronal and cardiac preparations. Aliment Pharmacol Ther, 2: 101-108, 1988.
- 11. Hills JM, Aaronson PI. Mechanism of action of peppermint oil on gastrointestinal smooth muscle. An analysis using patch clamp electrophysiology and isolated tissue pharmacology in rabbit and guinea pig. Gastroenterology, 101: 55-65, 1991.
- 12. Ishikawa M, Nakajima K, Ohno Y, Tanaka H, Kaneko K. Suppressive effects of NPO-11 on gastric motility. J New Rem & Clin, 59: 1845-1858, 2010. (in Japanese)
- 13. Taylor BA, Luscombe DK, Duthie HL. Inhibitory effect of peppermint oil and menthol on human isolated coli. Gut, 25: A1168-1169, 1984.
- 14. Mori A, Hachiya H, Yumura T, et al. L-Menthol sprayed on gastric mucosa causes edematous change. Endoscopy International Open, 2: E51-57, 2014.