Article ID: 2015-23
Article ID: 2015-23
We compared the effects of lysophosphatidylcholine (LPC) and acetylcholine (ACh) on IK(ACh), ICa and a non-selective cation current (INSC) in guinea-pig atrial myocytes to clarify whether LPC and ACh activate similar Gi/o-coupled effector systems. IK(ACh), ICa and INSC were analyzed in single atrial myocytes by the whole cell patch-clamp. LPC induced INSC in a concentration-dependent manner in atrial cells. ACh activated IK(ACh), but failed to evoke INSC. LPC also activated IK(ACh) but with significantly less potency than ACh. The effects of both ligands on IK(ACh) were inhibited by intracellular loading of pre-activated PTX. This treatment also inhibited LPC-induced INSC, indicating that IK(ACh) and INSC induced by LPC are both mediated by Gi/o. LPC and ACh had similar potencies in inhibiting ICa, which was pre-augmented by forskolin, indicating that LPC and ACh activate similar amounts of α-subunits of Gi/o. The different effects of LPC and ACh on IK(ACh) and INSC may suggest that LPC and ACh activate Gi/o having different types of βγ subunits, and that LPC-induced INSC may be mediated by βγ subunits of Gi/o, which are less effective in inducing IK(ACh).
Lysophosphatidylcholine (LPC) is an intracellular phospholipid metabolite that accumulates in the heart during ischemia1,2) and triggers lethal arrhythmia3-5). LPC is also a major component of oxidized low-density lipoprotein and has been proposed to play a role in the development of atherosclerotic lesions by stimulating endothelial cells, smooth muscles and blood cells6). LPC induces a non-selective cation current (INSC) in guinea-pig cardiac ventricular cells7) and in human vascular endothelial cells8). Recently, we found that LPC-induced INSC was mediated by activation of the pertussis toxin (PTX)-sensitive heterotrimeric G protein (Gi/o) as well as HMG-CoA reductase-dependent activation of the small GTP-binding protein, Rho9).
In atrial cells, the cholinergic M2 receptor is coupled with Gi/o and stimulation of this receptor with acetylcholine (ACh) induces an inwardly rectifying K+ current (IK(ACh))10). This effect on IK(ACh) is mediated by βγ subunits dissociated from α-subunits of Gi/o. On the other hand, α-subunits of Gi/o inhibit pre-activated adenylyl cyclase, thereby decreasing cyclic AMP-dependent responses. This effect is known as ‘accentuated antagonism’ and can be demonstrated by the ACh inhibition of L-type Ca2+ current (ICa) pre-augmented by β1-receptor stimulation11). Therefore, if LPC activates Gi/o, it may also activate IK(ACh) and inhibit ICa pre-activated by forskolin-induced cyclic AMP accumulation. In this study, using atrial cells, we compared the effects of LPC and ACh on IK(ACh) and ICa as well as INSC to examine whether the activation of Gi/o─mediated pathways by LPC and ACh utilize similar effector systems.
All experiments were performed in accordance with the regulations of the Animal Research Committee of Fukushima Medical University. Male guinea pigs weighing 250 to 400 g were anesthetized by intraperitoneal injection of 250 mg/kg sodium pentobarbital with 0.5 U/g heparin. The heart was removed and mounted in a Langendorff apparatus while perfusing with Tyrode solution and then with Ca2+-free Tyrode solution. After the heart stopped beating, Ca2+-free Tyrode solution containing 5~8 mg/50 ml collagenase (Wako, Tokyo, Japan) and protease (0.01 mg/50 ml, Nagase, Tokyo, Japan) was perfused for about 15 min. Then the solution was changed to a low Cl-- high K+ (KB or Kraftsbrühe) solution for 3 minutes. The atria were removed and shaken in KB solution to dissociate the cells. The cells were kept in KB solution at 4ºC.
SolutionsTyrode solution contained 140 mM NaCl, 5.4 mM KCl, 1.8 mM CaCl2, 1 mM MgCl2, 0.33 mM NaH2PO4, 5.5 mM glucose and 5 mM HEPES (pH 7.4 with NaOH). The low Cl-/high K+ solution (KB solution) contained 70 mM KOH, 50 mM l-glutamic acid, 40 mM KCl, 20 mM KH2PO4, 3 mM MgCl2, 0.2 mM EGTA, 20 mM taurine, 10 mM glucose and 10 mM HEPES (pH 7.2 with KOH). The pipette solution for measuring K+ currents contained 20 mM KCl, 120 mM KOH, 60 mM aspartic acid, 5 mM MgATP, 3 mM MgCl2, 20 mM BAPTA and 10 mM HEPES (pH 7.2 with aspartic acid). To measure K+ current, 5 µM nifedipine was added to the Tyrode solution to block ICa. The pipette solution for measuring ICa contained 130 mM CsCl, 20 mM TEA-Cl, 5 mM EGTA, 5 mM MgATP, 0.1 mM TrisGTP and 10 mM HEPES (pH 7.2). The external solution for measuring ICa contained 5.4 mM CsCl instead of KCl in the Tyrode solution to inhibit IK1.
Current recordingThe whole-cell voltage clamp method was employed using pCLAMP8 software (Axon Instruments, Foster City, CA, USA). Patch pipettes were fabricated with a two-stage microelectrode puller (pp83, Narishige, Tokyo, Japan) to form a tip diameter less than 1.5 µM, which had a resistance of 2~3 MΩ when filled with the pipette solution. The patch-clamp amplifier was Model TM-1000 (Act ME, Tokyo). Current signals were filtered by low-pass 2.5 kHz. The temperature of the bath solution was kept constant at 35~36ºC.
To record K+ current, ramp pulses of 480 msec duration were given at 10 sec intervals. The ramp pulse was initially depolarized from the holding potential of -40 mV to 60 mV, then hyperpolarized to -120 mV and depolarized back to the holding potential at a speed of 750 mV sec-1. The ICa was recorded by a step pulse of 200 msec duration from the holding potential of -40 mV to 10 mV every 10 sec.
DrugsAcetylcholine (daiichi pharmaceutical co., Ltd., tokyo, Japan) was dissolved in distilled water to make a 1 mM stock solution and was stored at -20ºC. LPC (L-1-palmitoyl-lysophosphatidylcholine, C16: 0) (Sigma, St. Louis, Mo., USA) was dissolved in methanol to make a 10 mM stock solution which was stored at -20ºC. Fluvastatin was a kind gift from Novartis (Basel, Switzerland). Forskolin (Sigma, St Louis, USA) was dissolved in DMSO to make a 10 mM stock solution and was stored at -20ºC. Pertussis toxin (PTX) (Sigma, St Louis, USA) (50 µg) was pre-activated by mixing with 5 mM dithiothreitol and incubating at 37°C for 15-20 min in 5 ml of the pipette solution12). The solution was diluted to 50 ml with the pipette solution to make a final concentration of pre-activated pertussis toxin, A-protomer, of 1 µg/ml. Anti-G protein β subunit (Gβ) antibody (SA-125) was obtained from BIOMOL (Plymouth Meeting, PA, USA). For intracellular loading of the anti-Gβ antibody, the patch pipettes were filled with a pipette solution containing 1: 1,000 diluted anti-Gβ antibody. All the chemicals used were the highest grade available.
Data analysisThe data were expressed as means±S.E.M. (number of cells) and analyzed by the Student’s t test. Differences with p<0.05 were considered significant.
Figure 1A shows representative current traces in response to 5 µM LPC added to the external solution. Soon after the application of LPC, the current increased slightly, and a marked increase appeared after about 2 min. Fig. 1B shows I-V curves obtained at the corresponding labels in Fig. 1A. The LPC-induced initial increase of the current crossed with the control current at about -70 mV (Fig. 1B a and b), indicating that the increased component was K+ current. The subsequent large LPC-induced current (c) crossed with the control (a) near 0 mV, suggesting that it was an INSC. Fig. 1C shows the concentration-response relationship between LPC and the INSC. The EC50 of LPC was 1.8 µM. There was a lag between the LPC application and the INSC development and the lag was shorter at higher concentrations of LPC (Fig. 1D). These results indicate that LPC induces INSC in atrial cells in a manner similar to that we reported previously in ventricular myocytes7,9).

Effects of LPC on membrane current of an atrial cell.
A, current recording versus time. Ramp pulse was given every 10 sec. LPC at 5 µM increased current slightly soon after application and significantly after a delay. B, I-V curves obtained at the corresponding labels in A. C, the concentration-response curve of a nonselective cation current (INSC) induced by LPC. The net INSC was obtained by subtraction (c-b) and the current density was calculated at -100 mV. The EC50 value of LPC was 1.8 µM. D, the relation between LPC concentrations and delay time before LPC began to induce INSC.
In the above experiment, a small inwardly rectifying K+ current was activated by LPC (Fig. 1 A-b, B-b). This would be an IK(ACh), because LPC activates Gi/o in ventricular cells9) and the IK(ACh) is activated by the βγ subunit of Gi/o in atrial cells13). To test this, we compared the effects of ACh and LPC on IK(ACh). In this set of experiments, LPC was applied for a short period of time of 30-60 sec to avoid the development of INSC. When 1 µM ACh was added to the bath solution, IK(ACh) was activated immediately (Fig. 2A). After washing out the ACh, application of 5 µM LPC induced a small inward current. I-V curves obtained with the control (a) and in the presence of 1 µM ACh (b) are superimposed in the left panel of Fig. 2B and those from another control (c) and in the presence of LPC (d) in the right panel of Fig. 2B. The net ACh-induced current (b-a) and the LPC-induced current (d-c) are superimposed in Fig. 2C. The average reversal potential of ACh-induced IK(ACh) was -70±3.5 mV (n=4) and that of LPC-induced current was -73±5.5 mV (n=4), values which were almost identical. The I-V curves of the LPC-induced current showed an inwardly rectifying property, supporting the view that LPC activated IK(Ach). Fig. 2D shows the relations between the current magnitude and the concentrations of ACh and LPC. The current density was measured at -100 mV. the K+ current induced by LPC was six times smaller than that induced by ACh, both at a concentration of 1 µM. This difference was not affected by reversing the order of application of ACh and LPC. Unlike LPC, ACh alone did not induce INSC during a prolonged application for 10 min (data not shown).
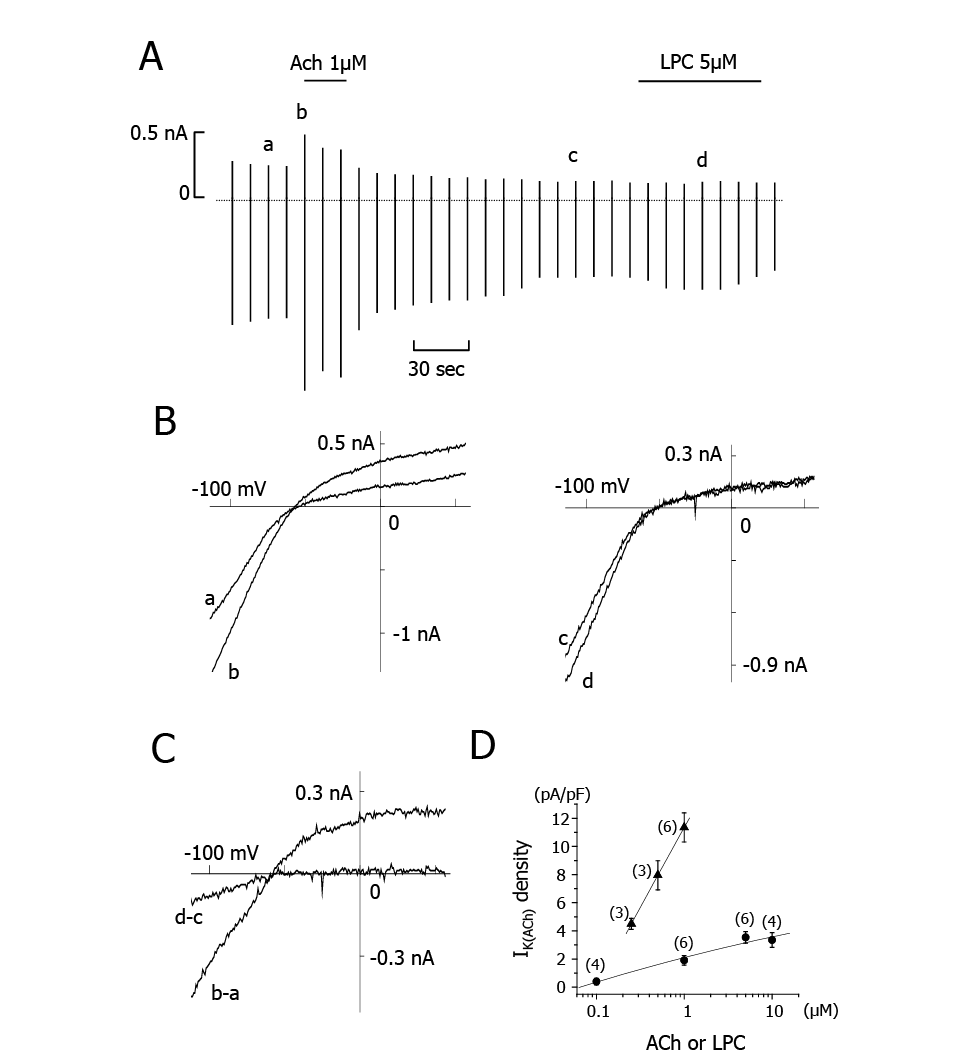
Effect of LPC on IIK(Ach).
A, Chart recording of currents. Applications of ACh and LPC are indicated by horizontal bars at the top of the figure. B, I-V curves obtained at the currents indicated by a~d in A. C, Difference I-V curves between control and peak with ACh (b-a), and in the absence and presence of LPC (d-c). The shape of the two I-V curves and the reversal potentials of b-a and d-c are similar. D, Concentration-response curves of ACh-(triangles)- and LPC- (circles) induced IIK(Ach). The IIK(Ach) densities were measured at -100 mV.
Activation of IK(ACh) by ACh is mediated by Gi/o10). We demonstrated previously that LPC-induced INSC is also mediated by Gi/o in cardiac ventricular cells9). Therefore, we tested whether the effect of LPC on the induction of IK(Ach) is also mediated by Gi/o. We added pre-activated PTX (1 µg/ml) in the pipette solution and allowed it to diffuse into the cell for 8~10 min. After confirming that PTX inhibited Ach-induced IK(Ach), LPC was added to the bath solution. As shown in Fig. 3, PTX completely abolished the LPC-induced IK(ACh). This result confirmed that the LPC-induced IK(ACh) was mediated by activation of Gi/o. PTX also abolished LPC-induced INSC.
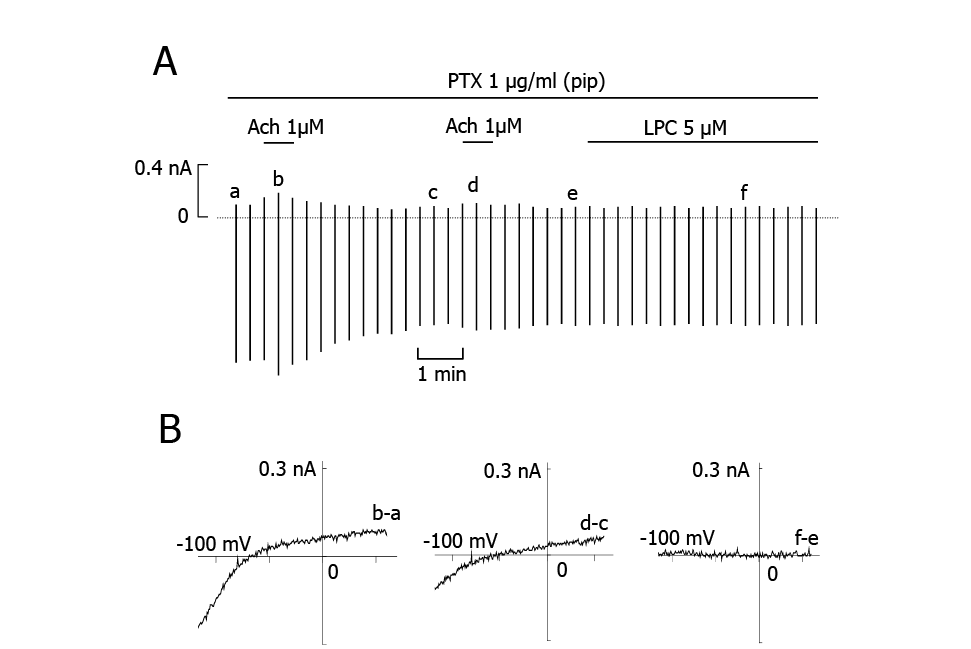
Effects of PTX on ACh- and LPC-induced IK(ACh).
A, Chart recording of currents. PTX was included in the pipette solution. After the response to ACh almost disappeared, LPC was added to the bath solution. B, Difference I-V curves from applications of ACh twice (b-a and d-c) and the application of LPC (f-e).
The above experiments demonstrated that both LPC and ACh induced IK(Ach) by activation of Gi/o via βγ subunits. We next investigated the functions of α subunits released from Gi/o proteins activated by LPC or ACh by measuring ICa pre-stimulated by forskolin, because ICa augmented by forskolin could be inhibited by Gi α subunits via inhibition of adenylyl cyclase (AC) which was pre-stimulated directly by forskolin (FK)15,16). As shown in Fig. 4Aa and 4Ab, ICa was increased to about 200~300% of the control by 3 µM FK. Subsequent application of 1 µM ACh reduced the FK-enhanced ICa (Fig. 4Ac). Finally, 5 µM nifedipine was added to inhibit Ica completely (Fig. 4Ad). The average value with standard deviation of the peak current amplitude of nifedipine-sensitive component of control ICa was 0.37± 0.19 nA (n=27), which was increased significantly by 3 µM FK to 0.71±0.55 nA (n=27). The p-value of ICa obtained by the paired T test was 0.0000002.
Using the same protocol, 1 µM LPC was added instead of ACh (Fig. 4B). Fig. 4C shows concentration-inhibition curves for LPC and ACh, where 3 µM FK-augmented ICa was calculated as the maximum of 100%. It is interesting to note that the inhibitory effects of LPC and ACh on FK-augmented ICa were similar over the concentration range from 0.1 µM to 3 µM. This result suggests that LPC and ACh activated similar amounts of α subunits of Gi/o. When the atrial myocytes were pre-treated with 2 µg/ml PTX for at least 3 h at 37ºC, the inhibitory effects of 1 µM ACh and 1 µM LPC on ICa were attenuated (data not shown), suggesting that the effects of ACh and LPC on ICa were both mediated by Gi/o.

Effects of LPC and ACh on ICa pre-enhanced by forskolin (FK).
A and B, Ca2+ current of the control (a), in the presence of 3 µM FK (b), with added 1 µM ACh (c in A) or 1 µM LPC (c′ in B) and further addition of 5 µM nifedipine (Nif) (d). The depolarizing pulses of 200 ms duration were given from the holding potential of -40 mV to 10 mV every 10 s. C, The concentration-inhibition curves of LPC (circles) or ACh (triangles) on ICa pre-stimulated by 3 µM FK. Number of cells examined are indicated in the parenthesis. Statistical significance was observed between the absence and presence of most of the concentrations of LPC or ACh by using Games-Howell test, which revealed p-values of 0.003, 0.004, 0.007 and 0.004 at 0.1, 0.3, 1 and 3 µM LPC, respectively. The p-values of 0.007, 0.88, 0.002 and 0.008 were obtained at 0.1, 0.3, 1 and 3 µM LPC, respectively.
The present study demonstrated that LPC induces INSC in a concentration-dependent manner in guinea-pig atrial myocytes. Possible schema of signal transduction is illustrated in Fig. 5. The LPC-induced INSC in atrial cells appeared after a time lag and was inhibited by PTX, which is similar to what we reported previously in ventricular myocytes9) and in cultured human aortic endothelial cells8). These results suggest that atrial cells possess LPC receptors, which are coupled to PTX-sensitive G protein. Recently, G-protein-coupled lipid receptors have been reported for LPC and other lipids, such as sphingosin-1-phosphate (S-1-P) and sphingosyl phosphorylcholine17). G2A18) and GPR419) have been reported as LPC receptors that are coupled with PTX-sensitive G protein. The expression of G2A is restricted to lymphoid tissues, whereas GPR4 is expressed more ubiquitously, including in heart and aorta19). Identification of receptor subtype that mediates LPC-induced responses in the atrial myocytes is remained to be determined.
The signal transduction mechanism underlying LPC-induced INSC is unique and complex, because it requires an HMG-CoA reductase-dependent activation of the small GTP-binding protein, Rho8,9). Although the underlying mechanism of Rho-INSC pathway is unknown, an activation of Gi/o plays an important role as the upstream regulator of Rho-dependent INSC activation because of blockade of this pathway by PTX9). In atrial cells, the activation of Gi/o stimulates IK(ACh) in a βγ subunit-dependent manner. Therefore, LPC might also activate IK(ACh). Indeed, a small inwardly rectifying K+ current developed soon after the application of LPC in atrial cells. This current is most likely IK(Ach), because the IV-curve of the LPC-induced K+ current was similar to that induced by ACh, and the current did not appear when PTX was in the pipette solution. However, LPC-induced IK(ACh) was significantly smaller than ACh-induced IK(ACh). In our result in Fig. 2, the outward component of LPC-induced current was minute, but this was not always the case (see Fig. 1B). We do not know the reason why there was a case that the outward component of LPC-induced IK(Ach) did not develop. Bünemann et al.20) also described insignificant effects of LPC on IK(ACh) in atrial myocytes.
Intracellular loading of anti-Gβ antibody prepared from C-terminal of Gβ subunit have been shown to inhibit βγ subunits-mediated effects selectively14). We attempted to investigate the effects of anti-Gβ antibody on LPC-induced INSC and IK(ACh). Our preliminary data indicated that intracellular loading of anti-Gβ antibody inhibited ACh (1 µM)-induced IK(ACh), as well as LPC (5 µM)-induced INSC or IK(ACh), suggesting that LPC-induced INSC and IK(ACh) are both mediated by βγ subunits of Gi/o proteins.
The results with ACh and LPC clearly show that activations of two different Gi/o-coupled receptors produce their effects through different effector systems, namely IK(Ach) and INSC. In cardiac myocytes, differential responses to activations of Gs family-coupled receptors were also demonstrated. Thus, β1-adrenergic receptors fully stimulate cyclic AMP signaling, including cyclic AMP accumulation, phosphorylation of phospholamban, Ca2+ channel activation and positive inotropic effects, whereas β2-adrenergic receptors failed to induce Ca2+ channel activation and positive inotropic effects. These differences are explained by a differential localization of receptors; β2-receptors are highly compartmentalized into the caveolae microdomain, but β1-receptors are more widely distributed throughout the plasma membrane. Different responses to LPC and ACh may be also explained by such receptor localizations. However, M2 receptor is demonstrated to be distributed widely in the cardiac cells similar to β1-receptor. Furthermore, unlike Gs-cyclic AMP signaling systems which are regulated by α subunit of Gs protein in the cardiac tissue, IK(ACh) and INSC are activated by the βγ subunits of Gi/o, which exist as varied isoform complexes. To date, five β-subunits (β 1~β 5) and twelve γ-subunits (γ1~γ12) have been cloned21). Wickman et al.22) tested 6 recombinant β- and γ-subunits and found that they activated IK(ACh) with various kact values ranging between 3 and 30 nM. the most potent combination was β2γ7 at 3.9 nM and the least potent was β1γ1 at 30 nM. Thus, it is the βγ subunits which determine the potency of the interaction with the effector, IK(ACh). It is therefore more likely that LPC and ACh may activate Gi/o proteins having different types of βγ subunits in guinea pig atrial cells.
Even if the effects of βγ subunits released are different between LPC and ACh, the effects of α-subunits of Gi/o may be similar. In cardiac myocytes, L-type Ca2+ current is enhanced by forskolin, which directly activates adenylyl cyclase (AC), and promotes phosphorylation of L-type Ca2+ channels by cAMP-dependent protein kinase16,23). On the other hand, α-subunits released from Gi/o inhibit AC and decrease forskolin-elevated cAMP, thereby blocking cyclic AMP-dependent activation of ICa, a phenomenon known as ‘accentuated antagonism’16,24). As shown in Fig. 4, ICa pre-stimulated by forskolin was inhibited to a similar extent by LPC and ACh over the concentration range between 0.1~3 μM. Since these effects were also inhibited by PTX, the inhibition of ICa by LPC and ACh were mediated by Gi/o. This indicates that the α subunits of Gi/o activated by LPC and ACh function in a similar manner. The AC isoforms expressed most abundantly in the heart are types V and VI which are both inhibited with similar potency by three different Giα isoforms; Giα1, Giα2 and Giα325,26). In addition, the Gi-related proteins, Gi1, Gi2, Gi3 and Go have been shown to inhibit AC in a PTX-sensitive manner27). These observations suggest that similar amounts of Gi/o are activated by LPC and ACh. Hashizume et al.28) reported that LPC at micromolar concentrations significantly decreased the heart rate and even stopped the beating of isolated perfused rat heart. This may be due to the effect of LPC on ICa and IK(ACh), although the effect on the former was larger than on the latter.
In conclusion, as shown in Fig. 5, we propose that LPC and ACh activate Gi/o having different βγ-subunits, which determines the potency of activation of IK(Ach) and INSC. Further study is necessary to identify the types of heterotrimer subunits of G proteins which are coupled with the receptors.
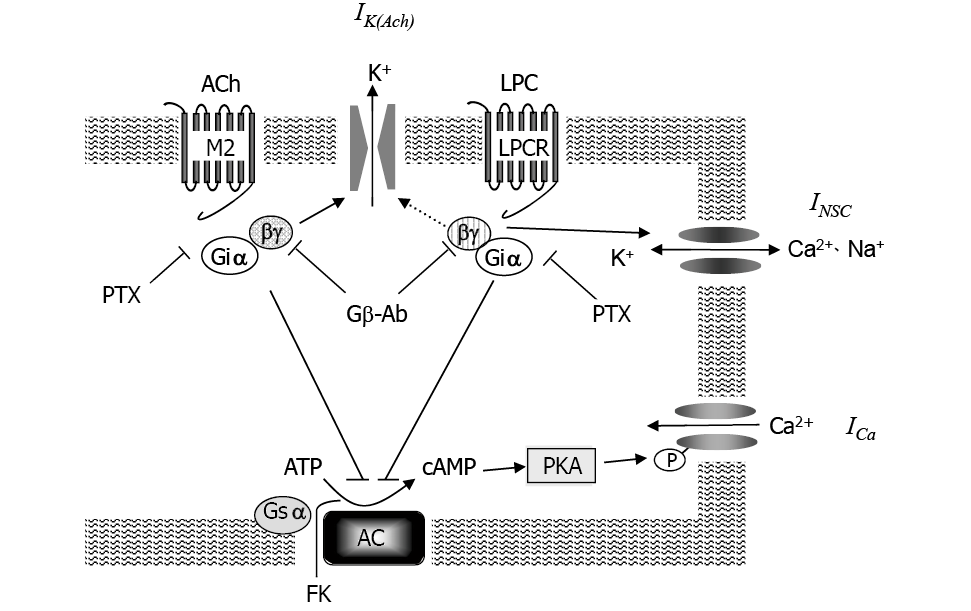
Proposed mechanisms of LPC and ACh effects.
Different βγ subunits are expressed with different shades. AC indicates adenylyl cyclase. FK indicates forskolin. LPCR indicates LPC receptor. Both ACh and LPC activate Gi protein with the same Giα subunits but different βγ subunits. Giα subunits activated by ACh and LPC inhibit FK-activated ICa by inhibiting AC. ACh-activated βγ subunit activates IK(ACh), while LPC-activated βγ subunit activates INSC. PTX inhibits Gi/o-mediated effects. Gβ antibody inhibited ACh-induced IK(ACh) and LPC-induced INSC by inhibiting βγ subunit function. M2: muscarinic receptor.
We thank Ms Sanae Satoh and Dr. Tomoyuki Ono for their technical assistance.
This work was supported by Grants-in-Aid for Scientific Research (13670092 and 11357020) from the Japan Foundation for Promotion of Sciences. Libing Li thanks the Nakajima Foundation and the Japan Rotary Club for scholarships for graduate studies.
We have no conflict of interest to disclose.