Article ID: 2018-05
Article ID: 2018-05
Background: To clarify the predictive factors for poor outcome in pediatric C3 glomerulonephritis (C3GN), we retrospectively evaluated the relationship between the clinico-pathological findings and prognosis in cases of pediatric C3GN. Methods: We enrolled 18 patients diagnosed with C3GN. These patients were divided into two groups, four patients in the end-stage renal disease (ESRD) group and 14 patients in non-ESRD group, based on clinical status at the last examination. Patients in the non-ESRD group were further divided into Subgroup A, consisting of 6 treatment responders, and Subgroup B, consisting of 8 non- responders. The clinical and laboratory findings, as well as the histological findings were investigated for each group. Results: The frequency of nephrotic syndrome at onset in the ESRD group was higher than that in the non-ESRD group. Before treatment and at 2 years after treatment, urinary protein excretion levels and serum creatinine levels in the ESRD group were higher than those in the non-ESRD group. The mean serum C3 and CH50 levels at 2 years after treatment in the ESRD group were lower than those in the non-ESRD group. The degree of renal injury, level of mesangial deposits and degree of alpha SMA staining at the time of the first renal biopsy in the ESRD group were all higher than those in the non-ESRD group. Conclusions: Our results suggest that the severity of C3GN at onset and persistent complements activity are associated with poor prognosis in C3GN.
C3 glomerulonephritis (C3GN), which forms a subgroup of C3 glomerulopathy (C3G), is a term coined for glomerular diseases characterized by the accumulation of complement C3 with the absence or only a trace amount of immunoglobulin deposition in the glomeruli and is the result of dysregulation in the alternative pathway of the complement system. Under light microscopy (LM), the majority of cases described show lesions typical of membranoproliferative glomerulonephritis (MPGN), although other histological patterns have been described. Electron microscopy (EM) has shown electron-dense, mesangial, intramembranous and/subendothelial deposits1-6). Servais et al. introduced the term C3G to describe isolated glomerular C3 without intramembranous deposits. Recently, the consensus meeting on C3G recommended that “glomerulonephtitis with dominant C3” should be used in practice as a morphological term for those cases with dominant staining for C3c with an intensity ≧2 orders of magnitude greater than that of any other immune reactant on a scale of 0 to 37-10).
Clinically, the disease presents with hematuria, proteinuria, and low serum C3 levels. The renal outcome for patients with C3GN is not as well established. The disease seems to have a chronic, but progressive course, with deterioration in renal function and development of end-stage renal disease (ESRD) observed in up to 50% of patients4,5,11,12). To date, reports on pediatric C3GN have indicated that the frequency of ESRD is about 0-20%4,5). Okuda et al. reported that C3GN may be more refractory than classical MPGN to immunosuppressant therapy11). In addition, we reported that the treatment response and prognosis of C3GN are worse than those of classical MPGN12). However, there have been no reports on the predictive factors for poor outcome in pediatric C3GN.
To clarify the predictive factors for poor outcome in pediatric C3GN, we retrospectively evaluated the relationships among the clinical and laboratory findings, pathological findings, and prognosis in cases of pediatric C3GN.
The study was carried out under the auspices of the Committee for Human Experiments at the Fukushima Medical University School (Institutional Review Board approval number 29238). Informed consent was obtained from all patients or their parents.
PatientsWe included data for 18 patients diagnosed with “C3GN based on the recommendations of the consensus meeting on C3 glomerulopathy” from among the patients who underwent renal biopsy at the Department of Pediatrics of Fukushima Medical University School of Medicine between 1974 and 2010. Entry criteria included: (1) diagnosis of C3 glomerulonephritis; (2) aged under 15 years of age at the start of therapy; (3) followed up for more than 5 years; (4) no previous treatment with corticosteroids or immunosuppressive drugs; and (5) sufficient renal biopsy tissue available for histological evaluation (minimum of 10 glomeruli). The age at onset (years) and male-to-female ratio of all patients were 10.4 ± 2.4 and 3: 16, respectively.
These patients were divided into the ESRD group (n=4) and non-ESRD group (n=14) based on clinical status at the last examination. The patients in the non-ESRD group were further divided into two subgroups: Subgroup A, consisting of 6 patients categorized as having normal urine or minor urinary abnormalities at the most recent observation, and Subgroup B, consisting of 8 patients categorized with persistent nephropathy. The epidemiological, clinical and laboratory findings, as well as the histological findings from the renal biopsy were investigated for each group.
DefinitionsPatients were defined as hematuria-positive if microscopic examination showed five or more red blood cells per high-power field, and macrohematuria-positive if visible with the naked eye. Proteinuria was evaluated by 24-hour quantitative measurement. Chance hematuria was defined as hematuria that was discovered by chance in school urinalysis or medical examination, and chance proteinuria was defined as proteinuria that was discovered by chance in school urinalysis or medical examination.
The estimated glomerular filtration rate (eGFR) was calculated based on the serum creatinine level and patient height13). A diagnosis of C3GN was based on the following findings under light microscopy: enlarged and lobular glomeruli, an increase in mesangial cells and matrix, and a double contour of the capillary loop that signified primary disease without systemic disease such as monoclonal gammopathy, anti-phospholipid antibody syndrome, lupus nephritis, other autoimmune diseases, hepatitis B or hepatitis C viral infection, or post-infectious glomerulonephritis with elevated ASLO and/or an episode of infection before onset. C3GN was used to describe the morphological condition in which cases showed dominant staining for C3c with an intensity ≧2 orders of magnitude greater than that of any other immune reactant on a scale of 0 to 34).
The clinical status of each patient was classified into one of the following categories of disease activity: Stage 1 (Normal urine); the results of the physical examination were normal, and the patient had normal urine and normal renal function, Stage 2 (minor urinary abnormalities); the results of the physical examination were normal, but urinalysis revealed microscopic hematuria or proteinuria of less than 20 mg/m2/h, Stage 3 (persistent nephropathy); the patient had 20 mg/m2/h or greater proteinuria, and e-GFR was 60 ml/min/1.73 m2 or greater, and Stage 4 (renal insufficiency); the patient had an e-GFR value of less than 60 ml/min/1.73 m2, with the ESRD patients in particular having an e-GFR value of less than 15 ml/min/1.73m2, including those on dialysis, undergoing transplantation or dead.
PathologyAn initial renal biopsy was performed for all patients. The mean (±SD) number of glomeruli found in the biopsy specimens was 18.9 ± 8.4 (range 11-28). The specimens were assessed by LM, IF and EM. The procedures for the histological examination, including IF, LM, EM and immunohistochemical detection, were performed as described in previous reports14,15). To compare the biopsy specimens, a histological scoring system was modified to evaluate acute and chronic changes. Acute changes (acute index) included capillary wall thickening (grade 0, normal; 1, slight; 2, moderate; 3, severe), mesangial proliferation (grade 0-3), capillary loop patency (0-3, where 0 indicates most loops were patent and 3 indicates most loops were closed), cellular crescent formation (scored according to the percentage of glomeruli involved: 0% = 0, 1-20% = 1, 20- 50% = 2, > 50% = 3), and interstitial mononuclear infiltration (scored according to the percentage of interstitial mononuclear infiltration: 0% = 0, 1-20% = 1, 20- 50% = 2, > 50% = 3). Chronic renal injury (chronic index) was estimated by determining the number of glomeruli showing fibrous crescents and segmental or global sclerosis. Each abnormality was scored on a scale of 0-3 according to the number of glomeruli involved as for acute crescent formation. In addition, the combination of tubular atrophy and interstitial fibrosis was graded on a scale of 0-3 (scored according to the percentage of tubular atrophy and interstitial fibrosis: 0% = 0, 1-20% = 1, 20- 50% = 2, > 50% = 3). The individual scores were added to obtain the acute index and chronic index.
As to scoring for the evaluation for macrophages (CD68) and alpha-SMA, the number of macrophages was defined as the number of cells positive for CD68 within the glomeruli. Positive cells were counted in all glomerular cross-sections in the biopsy specimens. The number of positive cells per glomerulus was graded according to the follow scale: 0=none, 1=1-5 cells per glomerulus, 2=6-10 cells per glomerulus, and 3=more than 11 cells per glomerulus. The degree of alpha-SMA expression in each glomerulus was graded according to the following scale: 0=negative staining, 1=week, segmental mesangial staining or periglomerular staining, 2=strong, segmental mesangial staining, and 3=strong, diffuse mesangial staining. The total expression score was divided by the number of glomeruli, and the average score was taken as the value for the patient. As to interstitial CD68 and alpha-SMA-positive cell infiltration, the numbers of cells per high-powered field (i.e., 400X) were counted. Tissues for histological study were reviewed by two independent investigators who were unaware of the clinical data for the patients in the study.
TreatmentPrior to 1984, patients were treated with “P” therapy following diagnostic renal biopsy. “P” therapy consisted of a combination of “pulse” methylprednisolone at 30 mg/kg/day i.v. bolus (maximum 1 g) for three consecutive days, followed by four weeks of daily oral prednisolone (1 mg/kg/day) and alternate-day prednisolone (1 mg/kg/day) for more than 23 months, together with dipyridamole (5 mg/kg/day) and warfarin. Warfarin was given orally as a single morning dose of 1 mg (less than 7 years) or 2 mg (more than 7 years) to maintain the thrombotest at 30 to 50%. After 1984, patients were treated with “P+C” Therapy. “P+C” Therapy consisted of “P” therapy plus cyclophosphamide at 2.5 mg/kg/day for 8-12 weeks. An angiotensin-converting enzyme inhibitor (ACEI) was administered to patients with persistent nephropathy and exacerbation of proteinuria.
StatisticsData are expressed as the mean values ± SD. The statistical analysis was performed using a Macintosh computer with a software package for statistical analysis (Version 4 of Stat View, Abacus Concepts, Berkeley, Calif., USA). Differences in the laboratory findings between the two groups were assessed using the “Mann-Whitney U test”. Correlations were evaluated using the Fisher r-test.
The numbers of patients diagnosed with C3GN during the period covered by the study was 18. The number of patients with C3GN from 1974 to 1979, from 1980 to 1984, from 1985 to 1989, from 1990 to 1994, from 1995 to 1999, from 2000 to 2004, from 2005 to 2009, was 5, 4, 5, 2, 1, 0, and 1, respectively. The age at onset (years) and male-to-female ratio were 10.0 ± 2.9 and 1 : 3, respectively, in the ESRD group and 10.6 ± 2.4 and 2 : 12 in the non-ESRD group. With regard to symptoms at onset (Table 1), chance hematuria and/or proteinuria was identified by school urinary screening (SUS) in one patient (25%) in the ESRD group and 13 patients (93%) in the non-ESRD group, acute nephritic syndrome was found in 2 patients (50%) in the ESRD group and one patient (7%) in the non-ESRD group, and nephrotic syndrome (NS) was found in 3 patients (75%) in the ESRD group whereas it was not identified in the non-ESRD group. As to the symptoms at onset, the frequency of chance hematuria and/or proteinuria in the ESRD group was lower than that in the non-ESRD group whereas the frequency of NS in the ESRD group was higher than that in the non-ESRD group (p< 0.05). All ESRD patients underwent dialysis, but none received renal transplantation. The mean duration from the onset of symptoms to dialysis was 9.5 ± 1.7 years. No patients underwent genetic examination for dysregulation of the alternative complement pathway. Comparison of laboratory data at the time of the renal biopsy among groups (Table 2)
Before treatment, urinary protein and hematuria were observed in all patients. The urinary protein excretion levels and serum creatinine levels in the ESRD group were higher than those in the non-ESRD group. There were no significant differences in serum albumin levels, the mean serum C3 values, serum C4 values, or serum CH50 values between the ESRD and the non-ESRD groups. e-GFR in the ESRD group was lower than that in the non-ESRD group. In the non-ESRD group, urinary protein excretion levels and serum creatinine levels in Subgroup B were higher than those in Subgroup A.
At 2 years after treatment, the urinary protein excretion levels and serum creatinine levels in the ESRD group were higher than those in the non-ESRD group, while e-GFR in the ESRD group were lower than those in the non-ESRD group. The mean serum C3 and CH50 levels in the ESRD group were lower than those in the non-ESRD group. In the non-ESRD group, there were no differences in serum albumin levels or the mean serum C3 or CH50 levels between Subgroup A and B.
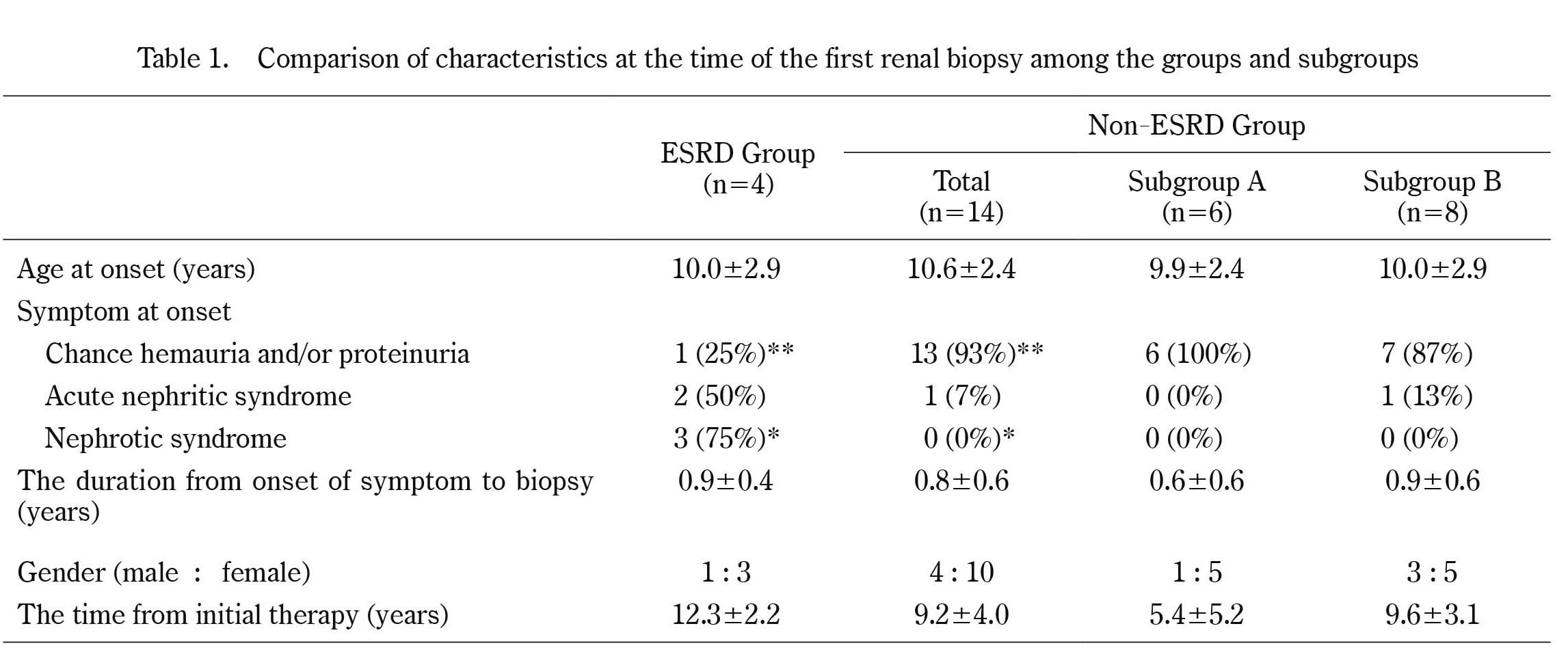
Comparison of characteristics at the time of the first renal biopsy among the groups and subgroups
* p<0.05, **p<0.01
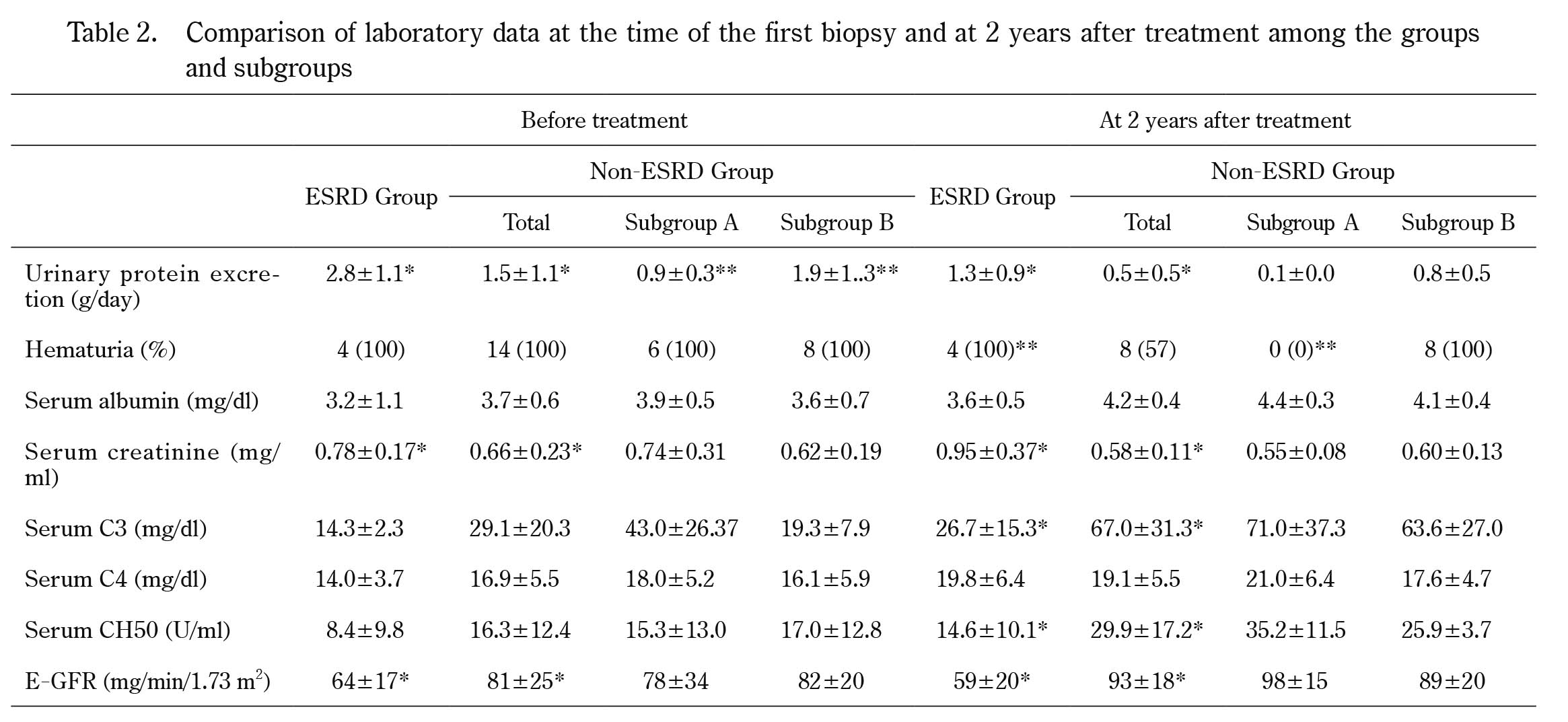
Comparison of laboratory data at the time of the first biopsy and at 2 years after treatment among the groups and subgroups
*p<0.05, **p<0.01
With regard to the IF findings at the first biopsy, there were no differences in the degrees of IgG, IgM, IgA, or C1q, C3, C4 complements deposition or fibrinogen deposition between the two groups.
With regard to the LM findings at the first biopsy, the acute index in the ESRD group was higher than that in the non-ESRD group (10.8 ± 4.1 vs. 7.4 ± 2.4 p< 0.05), and the chronic index in the ESRD group was higher than that in the non-ESRD group (3.3 ± 4.6 vs. 1.1 ± 1.3, p< 0.05) (Table 3, Figure 1).
With regard to the EM findings at the first biopsy, the score for mesangial deposits in the ESRD group was higher than that in the non-ESRD group; however, there were no differences in the scores for subepithelial deposits, subendothelial deposits or intramenbranous deposits between ESRD group and non-ESRD groups (Table 4).
Immunohistochemical findings in a patient with C3GN were showed in Figure 2. With regard to the immunohistochemical findings at the first biopsy, the glomerular α-SMA scores and interstitial α-SMA scores were higher in the ESRD group than in the non-ESRD group, and there were no differences in the glomerular CD68 scores or interstitial CD68 scores between the two groups (Table 4).
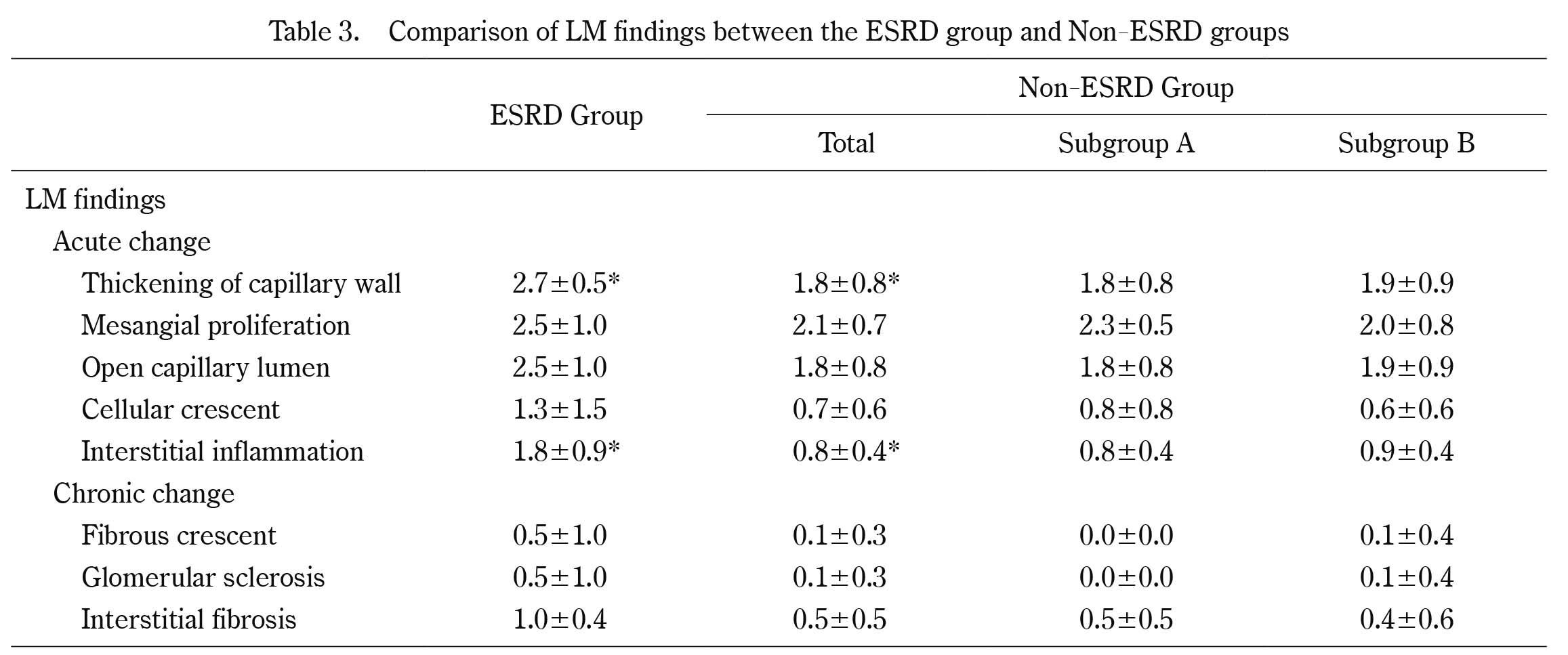
Comparison of LM findings between the ESRD group and Non-ESRD groups
*p<0.05

Comparison of AI and CI values among the groups and subgroups
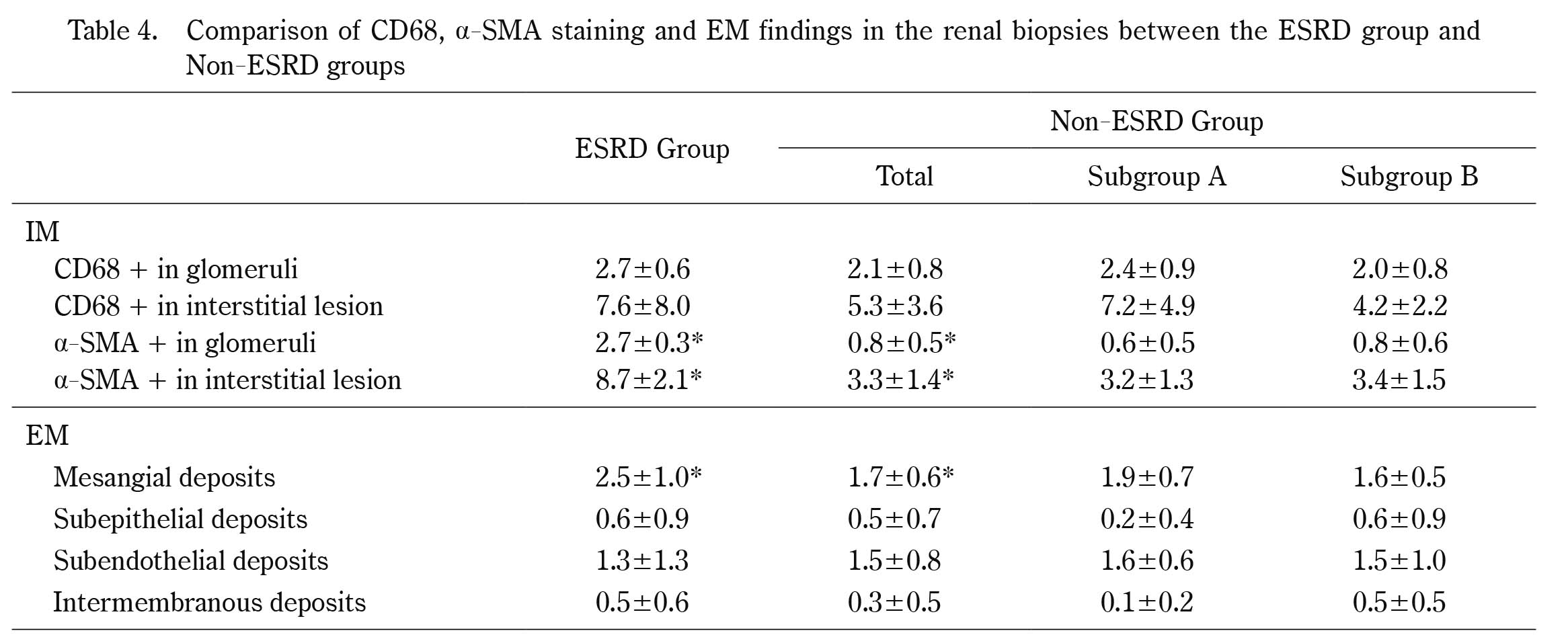
Comparison of CD68, α-SMA staining and EM findings in the renal biopsies between the ESRD group and Non-ESRD groups
*p<0.05

Immunohistochemical findings in a patient with C3GN
A: CD68-positive cells are observed in a glomerulus (X400).
B: CD68 positive cells are observed in interstitial lesions (X200).
C: α-SMA staining positive cells are observed in a glomerulus (X400).
D: α-SMA staining positive cells are observed in interstitial lesions (X200).
Therapy P was performed for 2 patients in the ESRD group and 7 patients in the non-ESRD group, whereas therapy P+C was performed for 2 patients in the ESRD group and 7 patients in the non-ESRD group. There were no significant differences in the therapy performed between the ESRD and non-ESRD groups.
C3GN describes a group of renal diseases defined by a specific renal biopsy findings with a dominant pattern of C3 fragment deposition under immunofluorescence examination. The primary pathogenic mechanism involves dysregulation of the alternative complement pathway. In addition, C3G can be divided into C3 glomerulonephritis (C3GN) and dense deposit disease (DDD), which is also known as MPGN type II4,5). The term C3GN was coined to describe glomerular lesions showing the glomerular accumulation of C3 with little or no immunoglobulin in the absence of the characteristic highly electron-dense transformation seen in DDD. Recently, the consensus meeting on C3G recommended that “glomerulonephtitis with dominant C3” should be used in practice as a morphological term for those cases with dominant staining for C3 above that than any other immune reactant7-10). However, there have been few reports to date on pediatric C3GN based on the new category recommended by the consensus meeting11). Further, the epidemiology, clinical and laboratory findings at onset, response to treatment, and pathological findings and prognosis of C3GN remain obscure. In Japan, there have only been two studies on the clinic-pathological findings of pediatric C3GN apart from case reports and, furthermore, this study is first report to deal with the poor prognostic factors for C3GN in Japan.
As to the epidemiology of C3GN, our results showed that the average age at onset was 10.4 years. The number of patients diagnosed with C3GN was 16 patients for the period from 1974 to 1992, and 2 patient from 1993 to 2010. The reason for the decrease in the number of patients diagnosed with C3GN during our observation period was unclear; however, we speculate that the incidence rate of C3GN may in fact be constant as one of the pathologic factors underlying C3GN is the dysregulation of the alternative pathway of the complement. Recently, it was reported that the incidence of MPGN and AGN have decreased due to changes in environmental factors including infection15,16). The reason for decrease in the number of patients with C3GN may be similar to that observed for MPGN and AGN. Thus, in terms of the pathologic factors underlying C3GN, we speculate that both changes in environmental factors as a trigger for its onset as well as the dysregulation of the alternative pathway of the complement are related to some degree.
With respect to the identification of symptoms at onset, 72 % of patients with C3GN in our study were identified by SUS, 17 % presented with NS, and 11 % presented with acute nephritic syndrome. Nicolas et al. also reported with regard to the clinical findings of C3GN at onset that 5 of 10 pediatric C3GN patients had recurrent hematuria, 2 had NS, and 3 had acute renal failure17). In our country, the Japanese Ministry of Education began a mass urine screening program for school children in 1974 that was aimed at the early detection of insidious renal diseases18). Thus, about 70% of our C3GN patients were identified by SUS. Further, of the 14 C3GN patients detected by SUS, 13 (93%) belonged to the non-ESRD group, with only one patient (7%) belonging to the ESRD group. Thus, almost all C3GN patients detected by SUS had non-ESRD. Furthermore, acute nephritic syndrome was found in 3 patients and NS was found in 3 patients. Two patients (67%) with C3GN presenting with acute nephritic syndrome and all patients with C3GN presenting with NS belonged to the ESRD group. The reason why the identification of chance urine abnormalities by SUS was lower in the ESRD group is thought to be that patients with ESRD often present with acute nephritic syndrome or nephrotic syndrome.
The pathogenesis of C3GN is thought to be associated with dysregulation of the alternative pathway of the complement. Potential abnormalities include genetic deficiencies in the regulators of the alternative complement pathway [such as complement factor (CF)H, CFHR1-5, CFI, and CD46] and autoantibodies against factor H, factor B, and C3 convertase1,5,19). As to the findings associated with the complement system in our study, all patients with C3GN had low serum C3 values and 16 patients (89%) had low serum CH50 values. These findings appear to confirm the notion that the pathogenesis of C3GN is associated with dysregulation of the alternative pathway of the complement. Furthermore, the mean serum C3 and CH50 levels at 2 years after treatment in the ESRD group were lower than those in the non-ESRD group. These results suggested that persistent complement abnormalities after combination treatment might be associated with prognosis in C3GN.
As to the pathological findings of C3GN, in the original French C3GN series reported by Servais et al., two main histologic groups were described. The first group had typical features of MPGN type 1, with mesangial proliferation, double contours, and subendothelial, mesangial, and (less commonly) subepithelial deposits. The second group lacked mesangial proliferation, the membranoproliferative pattern, and subendothelial deposits19). Sethi et al. described the clinicopathological findings in 10 cases of C3GN, and that reported eight cases showed MPGN with mesangial and endocapillary proliferation with mononuclear cells, glomerular capillary wall remodeling with double contour formation, and lobular accentuation of the capillary tufts, while the remaining two cases had diffuse endocapillary proliferative glomerulonephritis with endocapillary proliferation and an influx of both mononuclear cells and neutrophils19). In our study, all patients showed MPGN. The results of our examination revealed that AI and CI, including the scores for the thickening of capillary walls and interstitial inflammation, at the time of the first renal biopsy in the ESRD group were higher than those in the non-ESRD group. In addition, immunohistochemical findings and EM examination demonstrated that the score for alpha SMA staining and mesangial deposits in the ESRD group were higher than those in the non-ESRD group. These findings suggested that high AI and CI values, high levels of mesangial deposits, and strong alpha SMA staining at the first renal biopsy were associated with prognosis in C3GN. The high renal SMA scores and strong staining for mesangial deposits indicate that more immuno-complexes are deposited in the renal tissue and there are more activated mesangial transformed cells in the ESRD group than in the non-ESRD group, indicating that the inflammatory response in the kidney is strong. In addition, the fact that there was no difference in pathological histology between Subgroup A and B reveals that differences between the normal urine group and minor urinary abnormalities group could not be clarified on the basis of pathological histology at the first renal biopsy. According to the relationship between pathological findings and renal outcomes for MPGN, Schmitt et al. found that the prognosis of MPGN can be essentially determined by tubulointerstitial findings in the renal cortex21). Furthermore, Donadio et al. found a strong correlation between crescent formation and the progression of renal damage22). Our results were similar to those in previous reports with pathological findings being poor predictors of MPGN.
As to the treatment for C3GN, Nester et al. reported that anti-cellular immune suppression and plasma therapy have limited efficacy in cases of C3GN23). Rabasco et al. also reported on the effectiveness of mycophenolate mofetil in C3GN24). In our study, all patients were treated with therapy P or therapy P+C. One-third of the patients with C3GN had normal urine and about two-thirds of the patients had persistent nephropathy or renal insufficiency at the latest follow-up after combination therapy. The frequency of ESRD in patients treated with therapy P was similar to that in patients treated with therapy P+C. However, we are not able to comment on the efficacy of either treatment for C3GN. It is necessary to further evaluate the clinical manifestation, pathological findings and prognosis for C3GN patients according to treatment regimen.
There were several limitations to our study. First, the number of patients in this study was small as C3 nephropathy is a very rare disease and, therefore, multivariate analysis could not be performed. For the reason, we cannot discuss the factors without significant differences due to the small sample size. Finally, the present study was a retrospective study.
To further clarify the predictive factors for poor outcome in pediatric C3GN, it is necessary to undertake further evaluation based on a large-scale prospective clinical study.
Our results suggest that the severity of C3GN at onset and persistent complement activity are associated with poor prognosis in C3GN.