Article ID: 2018-13
Article ID: 2018-13
Background: Atrial septal defect is the third most common type of congenital heart disease. Surgical closure was the standard treatment for atrial septal defects before transcatheter closure was approved as minimally invasive treatment in Japan in 2006. In our hospital, this procedure has been performed since 2015.
Objective: To evaluate the safety and effectiveness of transcatheter closure of atrial septal defects in our hospital.
Methods: Thirty patients (mean age 57.0 ± 19.7 years, 11 males), who underwent transcatheter closure of atrial septal defect between September 2015 and February 2018 at the Fukushima Medical University Hospital, were enrolled. All procedures were performed under general anesthesia with angiographic and transesophageal echocardiographic guidance. Safety and effectiveness were evaluated by the procedural results and complications.
Results: All 30 patients successfully underwent transcatheter closure of atrial septal defects and no patient developed complications. New York Heart Association functional class was improved, and the right ventricular area and right atrium area were decreased, postoperatively.
Conclusion: Transcatheter closure is a safe and effective treatment for atrial septal defects, and thus could be an alternative option to open heart surgery.
Atrial septal defect is a common congenital heart disease and has two types: primum and secundum. Primum atrial septal defect is a variant of common atrioventricular canal defects. Secundum atrial septal defect is a defect that develops in the fossa ovalis due to the defective septum primum. Closure of atrial septal defects either percutaneously or surgically is indicated in patients with a hemodynamically significant shunt that causes enlargement of the right heart1).
Catheter intervention for structural heart disease has spread in recent years worldwide. Transcatheter closure of secundum atrial septal defects has been covered by public insurance in Japan since 2006. In Fukushima prefecture, transcatheter closure of atrial septal defects started from 2015 at Fukushima Medical University Hospital. We investigated the safety and effectiveness of transcatheter closure of atrial septal defects in our institution.
We enrolled 30 patients who underwent transcatheter closure of secundum atrial septal defects between September 2015 and February 2018. During this period, three patients were referred to our hospital due to atrial septal defects;however, they were not indicated for transcatheter closure because of small superior, inferior, or posterior rims by transesophageal echocardiography.
Baseline data including age, sex, body mass index, New York Heart Association class, past history, plasma levels of brain natriuretic peptide, echocardiographic findings, hemodynamic findings, and procedural data were collected.
Procedure of transcatheter closure of atrial septal defects is shown below. The implantation methods are similar between both devices of AmplatzerⓇ Septal Occluder (Saint-Jude Medical, Zaventem, Belgium) and FigullaⓇ Flex II ASD Occluder (Occlutech GmbH, Jena, Germany). All procedures were performed under general anesthesia with angiographic and transesophageal echocardiographic guidance. Before the procedure, we determined the size of occluder device by transesophageal echocardiography but not by a sizing balloon. First, a guide wire was inserted to the atrial septal defect through the right femoral vein. The occluder was screwed to the tip of the delivery cable, immersed in saline and drawn into the loader. A long guiding sheath and dilator were advanced over the guidewire through the atrial septal defect to the left atrium. After deployment of the left-sided disc in the left atrium, the system was pulled back. The occluder was thereafter fully deployed by withdrawing the sheath to expand the right atrial disc. Then, a residual shunt was evaluated by transesophageal echocardiography. Figure 1 shows an angiographic picture during the occluder deployment. The patients were discharged from the hospital 3 to 5 days after atrial septal occlusion. They received thienopyridine daily for a month and aspirin daily for 6 months. During the follow-up, blood sampling for brain natriuretic peptide measurement and transthoracic echocardiography were carried out at 1, 3, 6, and 12 months after atrial septal occlusion.
Data were analyzed using the Statistical Package for Social Sciences version 25 (SPSS Inc., Chicago, IL, USA). Continuous data are expressed as mean ± SD, and skewed data are presented as median and interquartile range. Categorical variables are expressed as numbers and percentages. The data were analyzed using Wilcoxon signed rank test. A P value of < 0.05 was considered statistically significant.
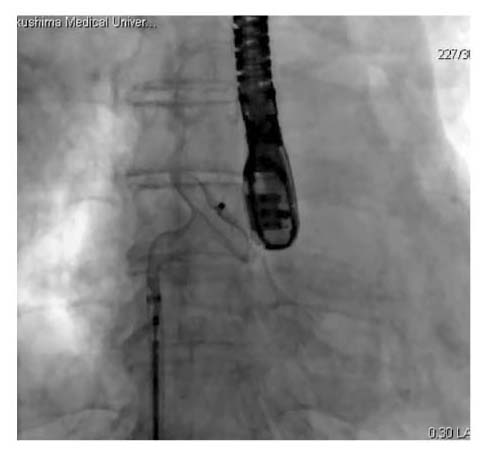
An angiographic image in transcatheter closure of atrial septal defect
The occluder was deployed by withdrawing the sheath to expand the right atrial disc.
The baseline characteristics of the 30 patients are shown in Table 1. The echocardiographic findings at baseline demonstrated preserved left ventricular systolic function, enlargement of right ventricle and both atria as shown in Table 2. The atrial septal defect diameter measured by transesophageal echocardiography was 18.6 ± 6.8 mm. The hemodynamic findings showed high mean pulmonary blood flow/systemic blood flow ratio of 2.30 ± 0.80. Five out of eight patients with atrial fibrillation underwent pulmonary vein isolation for atrial fibrillation before transcatheter closure of atrial septal defects.
All patients successfully underwent transcatheter closure. AmplazterⓇ Septal Occluder and FigullaⓇ Flex II ASD Occluder were used in 18 and 12 patients, respectively. No device-related complications were observed during the follow-up period of 312 ± 485 days (4 to 807 days) until April 2018 (Table 3). A slight residual shunt was detected immediately after atrial septal occlusion in all patients. However, among 22 patients, who underwent follow-up echocardiography 6 months after atrial septal occlusion, no residual shunt was detected. A septuagenarian patient with elevated brain natriuretic peptide of 506 pg/mL and mitral regurgitation before atrial septal occlusion needed additional diuretics treatment postoperatively.
After atrial septal occlusion, New York Heart Association class was improved (Figure 2). Right ventricular diastolic area, tricuspid valve annular diameter, and right atrium area were decreased (Figures 3, 4, and 5). Tricuspid regurgitation peak gradient and brain natriuretic peptide levels were not significantly changed (Figures 6 and 7) and echocardiographic images showed decreased size of the right ventricle and right atrium after atrial septal occlusion (Figure 8).
Three patients had pulmonary hypertension defined as mean pulmonary artery pressure of ≥ 25 mmHg. Two out of the three patients showed decrease in pulmonary artery pressure estimated by tricuspid regurgitation peak gradient of echocardiography 3 months after atrial septal occlusion. The remaining patient had residual pulmonary hypertension 6 months after atrial septal occlusion.
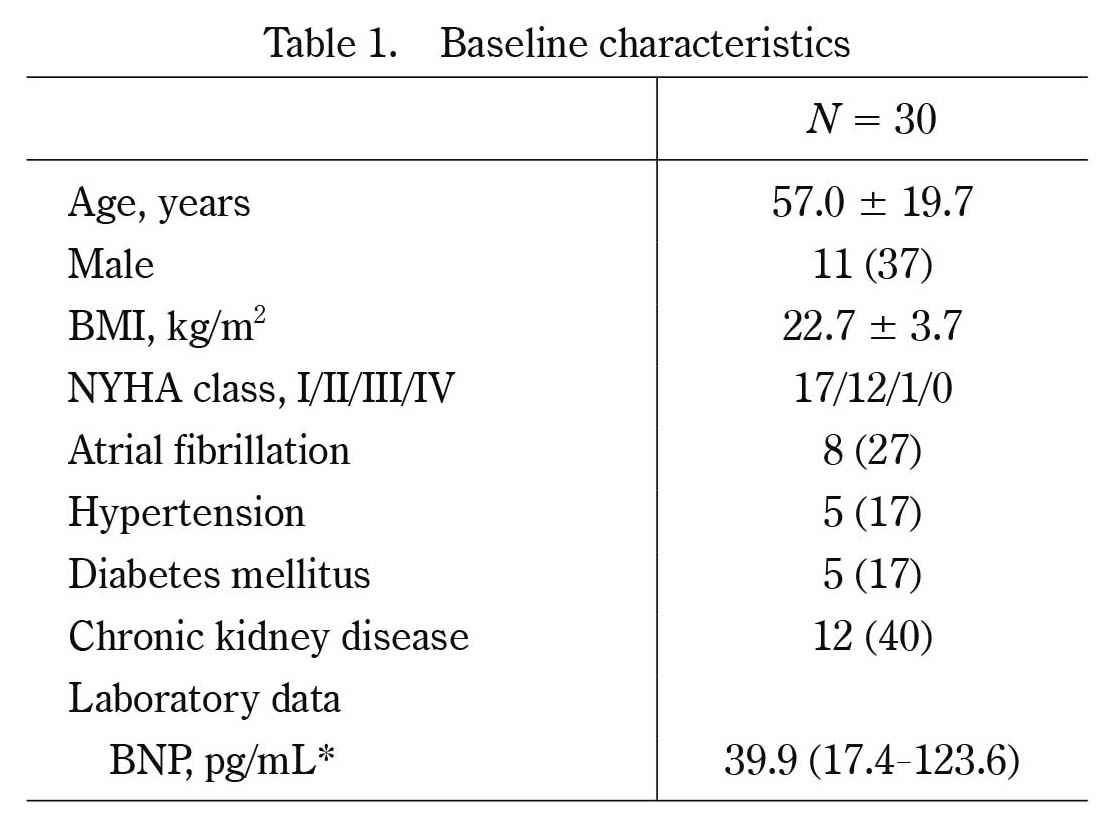
Baseline characteristics
Values are mean ± SD, *median (IQR), or number (%). BMI, body mass index ; NYHA, New York Heart Association; BNP, brain natriuretic peptide ; IQR, interquartile range

Echocardiographic and hemodynamic findings
Values are mean ± SD.
Qp/Qs, pulmonary blood flow/systemic blood flow ratio

Procedural results
Values are mean ± SD, or number (%).

Changes in New York Heart Association class after transcatheter closure of atrial septal defect
New York Heart Association class was decreased after atrial septal occlusion in many patients.

Changes in right ventricular diastolic area after transcatheter closure of atrial septal defect
Right ventricular diastolic area was decreased significantly after atrial septal occlusion. Data are presented as mean ± SD. *P < 0.01 vs. before.

Changes in tricuspid valve annular diameter after atrial septal occlusion
Tricuspid annular diameter was decreased significantly after atrial septal occlusion. Data are presented as mean ± SD. *P < 0.01 vs. before.
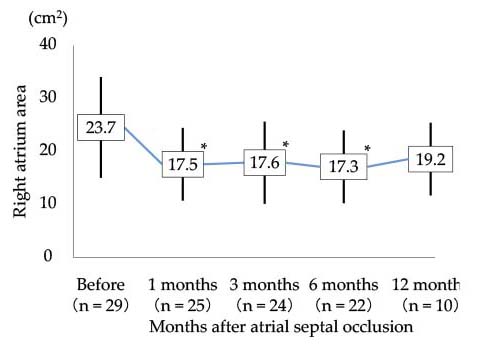
Changes in right atrium area after atrial septal occlusion
Right atrium area was decreased significantly after atrial septal occlusion. Data are presented as mean ± SD. *P < 0.01 vs. before.

Changes in tricuspid regurgitation peak gradient after atrial septal occlusion
Tricuspid regurgitation peak gradient did not significantly change. Data are presented as mean ± SD.

Changes in brain natriuretic peptide levels after atrial septal occlusion
Brain natriuretic peptide levels did not significantly change. Data presented are median and interquartile range.
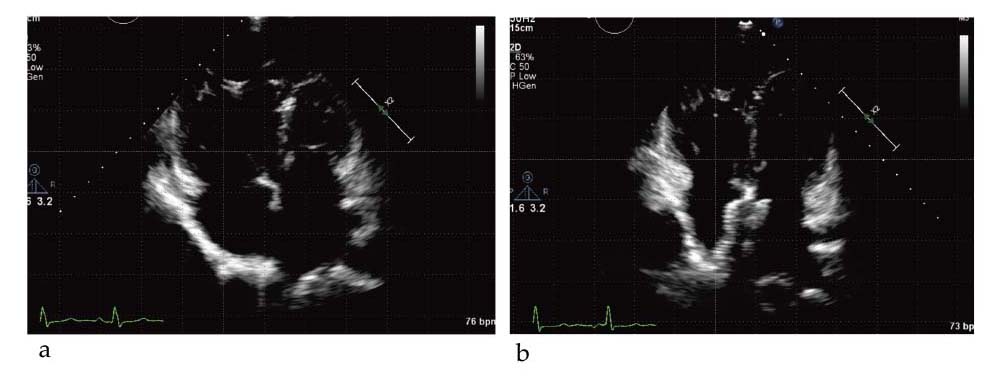
Representative echocardiographic images of before (a) and after (b) atrial septal occlusion
The 4-chamber echocardiographic images at end-diastolic phase show markedly decreased size of the right ventricle and right atrium after atrial septal occlusion.
Atrial septal defect is the third most common type of congenital heart disease, with an estimated incidence of 56 per 100,000 livebirths2). In most patients, atrial septal defects lead to a left-to-right shunt. The shunt results in impaired right atrial function, right ventricular dilatation, and pulmonary hypertension3). Most patients with atrial septal defect remain asymptomatic throughout most of childhood. Adult patients with large defects present with symptoms, including fatigue, palpitations, syncope, shortness of breath, edema, manifestations of thromboembolism, and cyanosis4).
First surgical closure for atrial septal defects under direct vision was performed in 19525). In 1997, Sharafuddin, et al. published an article on AmplatzerⓇ Septal Occluder for transcatheter closure of atrial septal defects6). Subsequently, Cowley CG, et al. reported advantages of this occluder such as fewer complications and shorter hospitalization than surgery7). Recently, the FigullaⓇ ASD Occluder has been developed and shown similar outcomes compared with AmplatzerⓇ Septal Occluder8).
In the majority of cases, transcatheter occlusion is safe and effective;however, complications associated with this procedure for atrial septal defects have been reported. The reported incidence of complications ranges from 0% to 9.4%9-11), including device embolization, device malposition, and cerebrovascular events.
In Fukushima Prefecture, transcatheter closure of atrial septal defect has been approved only at Fukushima Medical University Hospital. The procedural results in the present study had no complication, showing the safety of the procedure. Echocardiographic data demonstrated a decrease in the right ventricular area and right atrium area, suggesting that atrial septal occlusion improved volume overload of the right heart.
A septuagenarian patient with mitral regurgitation and elevated brain natriuretic peptide needed additional diuretics treatment after atrial septal occlusion. We should be careful of left-sided volume loading after atrial septal occlusion.12)
A limitation in this study is that although the incidence of late complications after transcatheter atrial septal occlusion is low, long-term follow-up is needed to verify the safety. A previous case report showed erosion 4 years after atrial septal occlusion13). Another case report described device thrombosis that occurred 7 years after atrial septal occlusion14).
In conclusion, transcatheter closure is a safe and effective treatment for atrial septal defect.
Tetsuro Yokokawa belongs to endowed departments supported by Actelion Pharmaceuticals Japan Ltd. Akiomi Yoshihisa belongs to a department supported by Fukuda Denshi Co, Ltd.