論文ID: 2020-05
論文ID: 2020-05
We encountered a case of pulmonary thromboembolism, in which an 84-year-old woman (body weight 62 kg, height 150 cm) fell in the ward eight days after upper arm surgery. In this event, she had fractured her ankle and hit her head, with transient loss of consciousness. She needed surgery for the ankle fracture under general anesthesia. Her anesthesia course was unstable, with heart rate varying between 95 and 140 bpm, systolic blood pressure between 70 and 110 mmHg, and oxygen saturation between 92 and 98%. Immediately after reversing anesthesia, we performed bedside ultrasound and diagnosed acute pulmonary embolism in the operating room. We assume that the event was not a simple fall, but pulmonary embolism-related fainting (syncope). This case and recent reports provide two lessons: (1) cases of syncope among postoperative patients may be reported as simple falls in the safety surveillance of hospitals, and (2) ultrasonography at the bedside plays a pivotal role in the diagnosis of pulmonary embolism in perioperative settings.
Falls are among the most commonly reported safety incidents in hospitals1). Elderly inpatients are at high risk because of comorbidities, gait instability, frailty, agitation and/or confusion in an unfamiliar hospital setting1). Although exact prevalence of fainting (syncope) is not reported, elderly inpatients have also an increased risk of syncope due to age-associated cardiovascular and autonomic changes and comorbidities2). Guidelines point out that falls and syncope often overlap in aged people and are often difficult to distinguish from each other3). We report a case of pulmonary thromboembolism (PE) diagnosed with point-of-care (POC) ultrasonography, i.e., ultrasonography performed and interpreted by physicians at the bedside, using a compact ultrasound device on a patient immediately after surgery for an ankle fracture. Written consent for publication was obtained from the patient and her family.
An 84-year-old-woman underwent a non-elective surgery because of an ankle fracture. Her weight and height were 62 kg and 150 cm, respectively, with body mass index of 27.6 kg/m2. She had left upper arm surgery eight days earlier in the same hospital. She fell in the bathroom while on the ward during the early morning two days previously. She hit her head and fractured her right ankle. She was unconscious when she was found by a nurse, but soon regained consciousness. She was not aware that she fainted. Her oxygen saturation (SpO2) was 88%, but increased to 94% with oxygen via a mask, with a blood pressure of 105/59 mmHg and heart rate 95 bpm. Although subsequent computed tomography (CT) of the head confirmed traumatic subarachnoid hemorrhage, with a small amount of blood in a region of the occipital sulci, no therapeutic intervention was deemed necessary for the head injury. However, she needed surgery for open reduction and internal fixation of the ankle fracture. She had atrial fibrillation, tachycardia of 120 bpm, diaphoresis and fever, and looked unstable at presentation before induction of anesthesia in the operating room (OR), although she responded appropriately to questions.
The surgery was performed under general anesthesia using desflurane and air combined with femoral triangle block and sciatic nerve block through a popliteal approach. Her airway was secured with a supraglottic device (#4 i-gel, Japan Medicalnext Co., Osaka). The surgery took one hour. Her anesthesia course of two hours and five minutes was unstable. Her heart rate varied between 95 and 140 bpm, systolic blood pressure varied between 70 and 110 mmHg, and frequent intermittent administration of phenylephrine was needed. Her SpO2 was 92-98%, though FIO2 was maintained at 50%. Her body temperature was 38.5℃. After restoration of spontaneous respiration and confirming eye-opening to verbal stimuli, we removed the supraglottic device. She looked pale, although her SpO2 was 94% with an oxygen mask at 4 L/min. Because of her unstable hemodynamics and unexplained low SpO2 during and after surgery, we performed POC ultrasonography with a portable device (SonoSite M-Turbo, Fujifilm, Tokyo) using a sector probe (SonoSite, P21x) to rule out disorders in the heart and lung.
Lung sliding was identified on both lung surface, excluding possibility of pneumothorax. We found no fluid collection in the thorax. The apical four-chamber view revealed, however, a dilated right ventricle (RV), with reduced free wall motion of the RV with normokinesis of the apical RV wall (McConell’s sign) and a moderate tricuspid regurgitant (TR) jet. The peak TR pressure gradient was 41 mmHg. Further, we confirmed a D-shaped left ventricle (LV) during diastole in the parasternal short axis view along with a round-shaped dilated inferior vena cava in the subcostal short axis view. Ultrasonography did not detect any thrombus in the right heart or in the femoral veins. These images were not stored in the device, because the examination was not part of a routine work-up. Because there were no such abnormalities in the previous recordings of echocardiography before the first surgery, we diagnosed an acute RV overload, presumably resulting from a PE. We urgently ordered multidetector CT. CT angiography demonstrated filling defects in the right and left pulmonary arteries with an RV/LV diameter ratio of 1.5 (Figure 1A, B). CT venography showed only a small thrombus in the right popliteal vein and no residual thrombus in the greater veins such as the popliteal, femoro-iliac, or inferior vena cava. Her SpO2 was thereafter stable above 97% with oxygen supplementation via face mask for the following two days. She was carefully managed by cardiologists with oral anti-coagulant therapy (edoxaban). She was discharged from the hospital two weeks later.
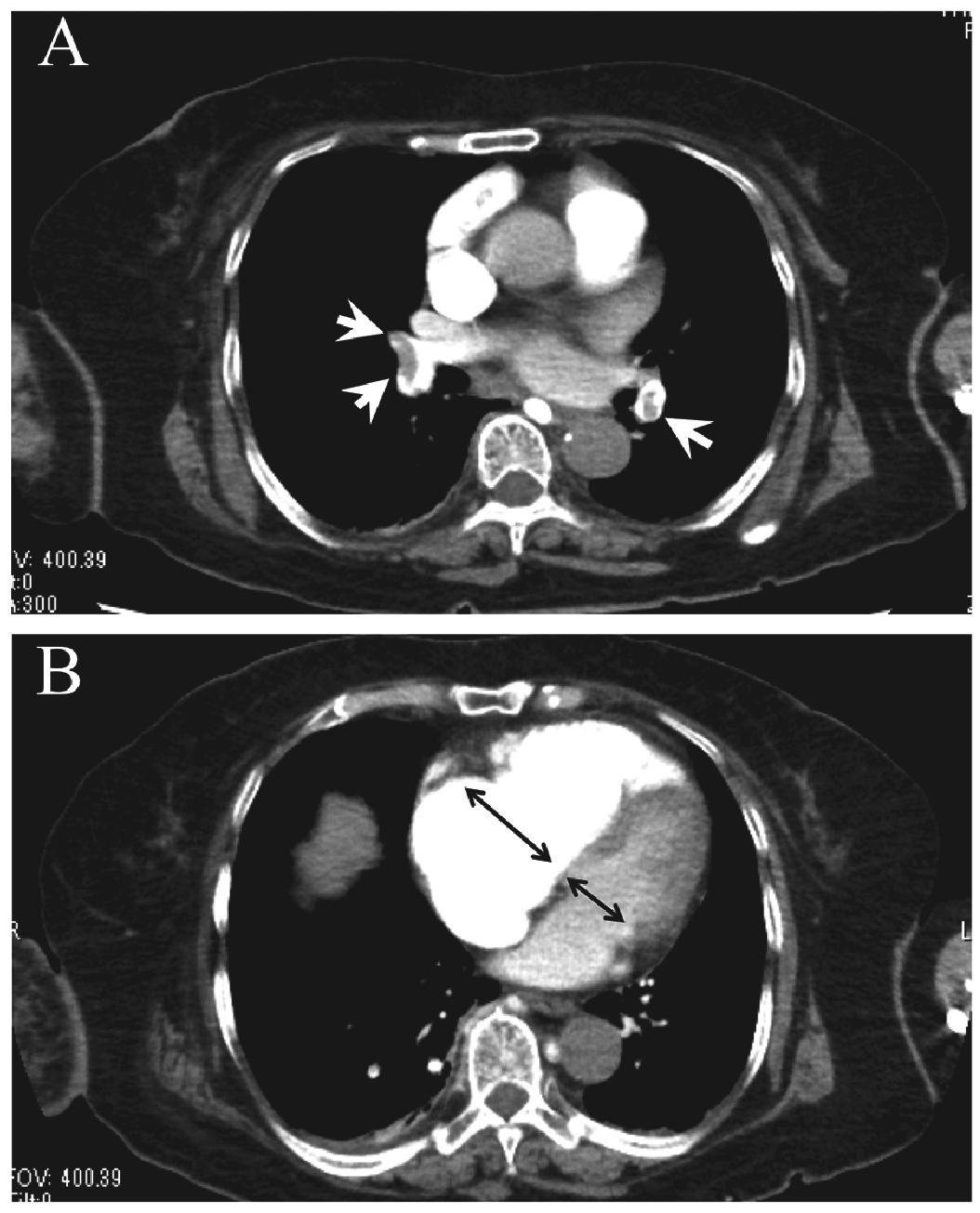
CT angiography of the chest.
A: Filling defects in the right and left pulmonary arteries are shown in the CT angiography (white arrow heads).
B: CT angiography showed a markedly dilated right ventricle. The ratio of the diameter of the right ventricle to that of the left ventricle is 1.5 (black arrows), indicating severe right ventricular dysfunction.
We presented a case of PE that was diagnosed with POC ultrasonography in the OR. In this instance, she had fainted, although medical staff and the patient herself believed that she had fallen. We speculate that PE was the underlying cause of the patient’s syncope, resulting in fall-related head injury and ankle fracture.
According to guidelines, syncope in the elderly often presents with falls and injuries, as with this case3). She had no known risk factors for postoperative deep vein thrombosis except for advanced age of 84 years. Since falls are not rare among orthopedic inpatients and she was not in circulatory collapse at the event, we initially considered that the event was a simple fall. We attributed temporal loss of consciousness to head injury.
With exception of a severe form of PE presenting as a sudden onset of life-threatening shock, PE is usually characterized with nonspecific symptoms such as dyspnea, fatigue, tachypnea, high fever, diaphoresis, tachycardia, and chest pain4). Thus, it is often difficult to diagnose it in a timely manner even in postoperative patients, despite of the fact that PE is a well-recognized postoperative complication.
Etiologically, syncope can be neurally mediated, have cardiac causes, and/or arise from orthostatic hypotension, with cardiac syncope having the worst mortality3). PE-related syncope is a type of cardiac syncope. Whereas a large thrombus obstructs the most proximal pulmonary arteries and results in a sudden decrease in cardiac output, thus resulting in cerebral hypoperfusion, smaller thrombi may also elicit a transient decrease in cardiac output via vasodepressor or cardioinhibitory mechanisms and arrhythmias, which may occur during the passage of a thrombus into the right heart5). In this case large thrombi were obviously responsible for PE, as shown in the CT angiography (Fig. 1A).
Although D-dimer testing is used initially to rule out PE in patients in the emergency department, it has only a limited value in the perioperative setting because of its low specificity in those patients4,6). On the other hand, POC ultrasonography plays a key role to rule in or rule out the diagnosis of PE in hemodynamically unstable patients with high-risk PE4). A recent meta-analysis confirmed that echocardiography has a sensitivity of 53% and specificity of 83%, even if the “right heart strain” sign, the most common, but not-well defined sign, is used7). Echocardiographic signs of PE are divided into three groups: 1) acute RV overload-related signs: RV-dilatation, hypokinesis of the RV free wall, interventricular flattering (D-shaped left ventricle), high TRPG (>30 mmHg) and distended inferior vena cava (IVC) without respiratory change; 2) RV dysfunction-related signs: RV free wall hypokinesis plus the RV/LV end-diastole diameter ratio greater than 0.9 measured in the apical four chamber view;and 3) typical echocardiographic signs (TES) for PE: “60/60” sign, McConell’s sign, and direct visualization of RV thrombus by Kurnicka et al.8), as shown in Table 1. According to their study, one TES plus RV overload-related signs were found in all hemodynamically unstable PE patients, although the “60/60” sign, McConell’s sign, and direct visualization of right heart thrombus were positive in 75%, 31.2%, and 18.8%, respectively, in those patients. We think, therefore, that positive findings of acute RV overload and one TES in our POC ultrasonography are enough to establish the diagnosis of PE and to avoid false diagnosis of acute PE in hemodynamically unstable patients and to proceed to multidetector CT for the definitive diagnosis of PE/DVT. Furthermore, it has to be mentioned that POC ultrasound is also useful to detect alternative causes of unstable hemodynamics such as pneumothorax, hypovolemia, pericardial effusion, valvular lesions, and LV dysfunction.
The differential diagnosis of echocardiographic acute RV overload/dysfunction includes acute right ventricular myocardial infarction (RMI) and undiagnosed chronic RV overload such as atrial septal defect or chronic PE. Acute RMI may reveal an echocardiographic pattern similar to McConell’s sign8), but its manifestation without chest pain/discomfort and echocardiographic normokinesis of the LV inferior segmental wall and absence of RV overload findings may be a clue for the differential diagnosis from acute RMI. Chronic RV overload is usually associated with a thickened RV free wall (>7 mm). In this case, we could easily exclude the chronic RV overload based on her normal echocardiographic findings two weeks earlier. In addition, echocardiography plays a pivotal role for risk stratification of PE4,9) and for the management of PE patients.
Fortunately, ultrasound devices are ubiquitous in modern operating rooms. Because anesthesiologists are using them routinely for nerve blocks and central venous cannulation and are thus familiar with handling these devices, we need to increase our diagnostic capability with POC echocardiography for the improvement of patient care in the OR.
In conclusion, this case provided two lessons: first, that PE could manifest as a fall/syncope in postoperative patients, and second, that POC ultrasonography is an appropriate first step to the diagnosis of PE in such hemodynamically unstable cases in the perioperative settings.
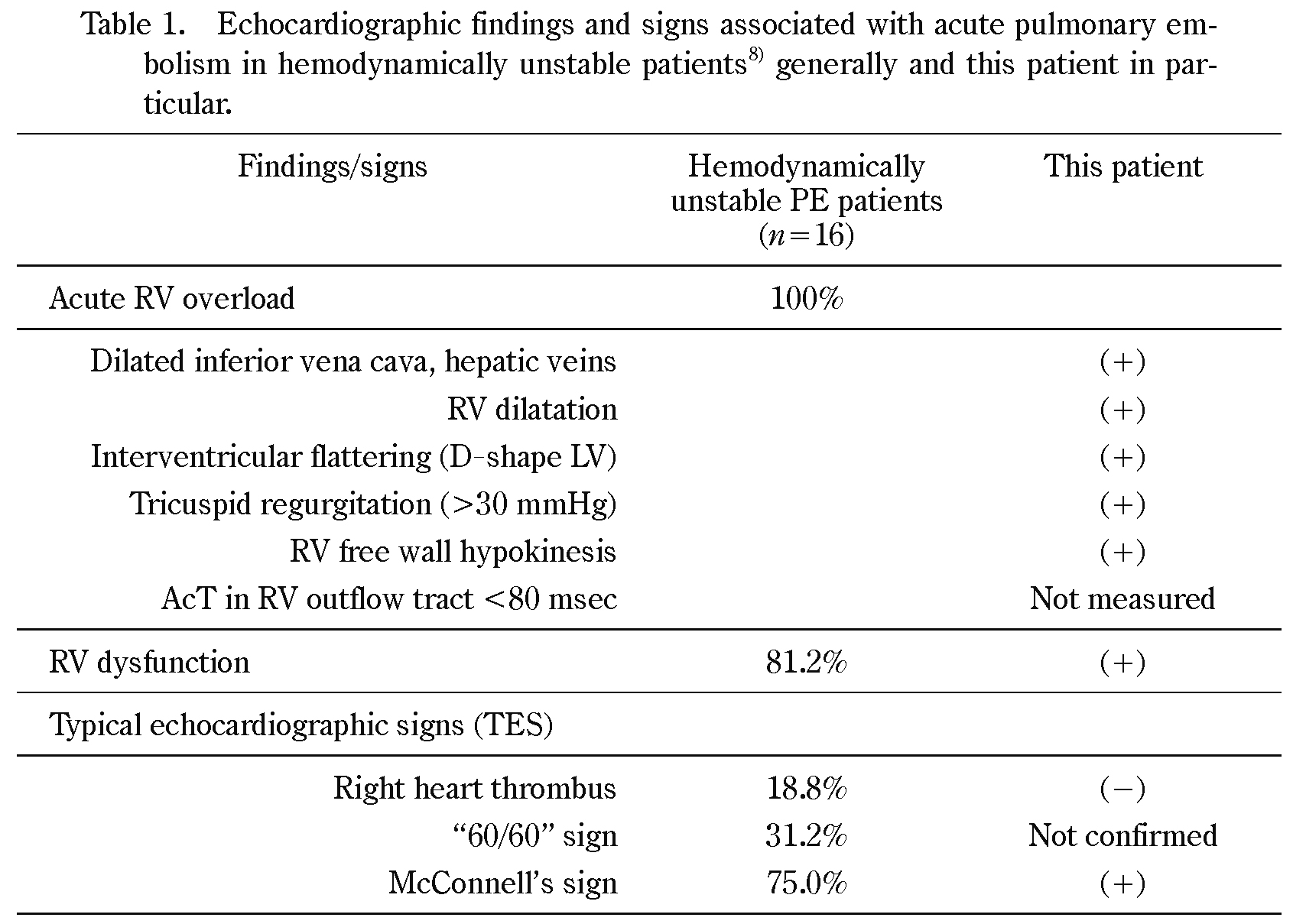
RV overload is defined as the condition that meets at least one of the listed findings. RV dysfunction is defined as RV free wall hypokinesis plus an end-diastole RV/LV diameter ratio greater than 0.9 measured in the apical four-chamber view. AcT in RV outflow tract <80 msec: Pulmonary ejection acceleration time measured in the RV outflow tract with pulsed-wave Doppler, indicating a high pulmonary vascular resistance (normal value >130 msec). Right heart thrombus: Visualization of thrombus in the right heart. “60/60” sign: pulmonary artery acceleration time ≤60 msec in the presence of maximal tricuspid regurgitant pressure gradient ≤60 mmHg. McConnell’s sign: RV free wall hypokinesis with normokinesis of the right apical segment.
Kurnicka et al.8) analyzed echocardiographic patterns of 511 consecutive PE patients confirmed by multidetector CT. Percentage of positive finding in patients with hemodynamically unstable PE are given. Although positive findings of TES vary considerably from 18.8 to 75%, any one of them and signs for RV overload were observed in all patients with hemodynamically unstable PE. Their study therefore suggests that one positive finding of TES plus signs of RV overload are the most useful echocardiographic criterion for the diagnosis of PE.
No financial support was received for this manuscript. The authors declare no conflicts of interest.