Article ID: 2020-08
Article ID: 2020-08
During wound healing, fibroblasts proliferate from the margin, and migrate into the provisional matrix where they differentiate into myofibroblasts resulting in wound contraction; however, fibroblasts are hyperproliferative during chronic tissue damage. We previously reported that cesium chloride inhibited a human cancer cell proliferation; therefore, cesium is also presumed to suppress fibroblast proliferation. We here investigated the effects of cesium chloride on the proliferation and migration of murine embryotic fibroblast cells, NIH/3T3 cells. Cultured NIH/3T3 cells with 0-10 mM sodium and cesium chloride were counted using trypan blue dye-exclusion method, then cell growth and viability were evaluated. The percentage of wound closure was calculated by scratch assay. The number of the cells was decreased by application of 1-10 mM cesium in a dose-dependent manner, whereas the viability of the cells was unchanged. The treatment with 3-10 mM cesium inhibited the proliferation rate and % of wound closure compared with controls. These results suggested that cesium inhibits the proliferation and migration of fibroblast cells. This study indicates a possible therapeutic role of cesium chloride in the treatment of wound healing and fibrosis.
Early in the wound repair process, dermal fibroblasts proliferate from the margin and migrate into the provisional matrix, composed of fibrin scaffold supporting the nascent blood clot. About 1 week later, granulation tissue, consisting of small vessels, the extracellular matrix (ECM) and fibroblast cells, replaces the provisional matrix. During granulation tissue formation, fibroblasts become activated and modulate into myofibroblasts. These differentiated myofibroblasts close the wound through contraction1).
Fibrosis is a pathological feature of disease in virtually all organs, such as the liver, kidney, heart, lung, joints, bone marrow, pancreas, and skin. It is associated with excessive deposition of ECM and leads to impairment of organ function. The disease is provoked by severe initial injury to the epithelium. Fibroblasts play a crucial role in fibrosis and are also related to wound healing processes2-4). After tissue injury, fibroblasts differentiate into myofibroblasts and contribute to tissue repair during the wound healing1). The fibroblasts become activated to migrate into the damaged tissue and to synthesize ECM. Excessive deposition of ECM is characteristic of fibrotic lesion, and it is known that fibroblasts are a source of ECM in the fibrotic lesions. Concerning wound healing and organ inflammation, adequate fibroblast proliferation is important in the healing process; however, its overgrowth leads to progression of chronic diseases such as fibrosis.
Cesium (Cs) is a member of the alkali metal elements. There is no evidence regarding the necessity of Cs in animal metabolism, and it induces toxicity at high concentrations5). Cs accumulates in the human liver, kidneys, and brain after intravenous administration. The half-life of Cs in a human male adult ranges from 50-150 days6-8). An application of Cs to the human cervical cancer cell line, HeLa cells, showed that its proliferation was suppressed by Cs in a dose-dependent manner9). Cs effects on cell proliferation are thought to involve intracellular metabolism; e.g., Cs suppressed pyruvate kinase activity. Pyruvate kinase is a glycolysis enzyme catalyzing conversion of phosphoenolpyruvate to pyruvate. Extracellular Cs was incorporated into intracellular spaces, then, the incorporated Cs competed with cytoplasmic potassium, which is required for pyruvate kinase activity10).
The suppression of fibroblast overgrowth may be brought about by an adequate healing process; therefore, we hypothesized that application of Cs is effective in suppression of fibroblast proliferation. In the present study, we evaluated the effect of Cs on murine fibroblast NIH/3T3 cell proliferation and whether Cs was able to suppress the fibroblast proliferation or not.
Murine NIH/3T3 fibroblast cells were cultured in growth media consisting of high glucose Dulbecco’s Modified Eagle Medium (DMEM, FUJIFILM Wako Pure Chemical Co., Osaka, Japan) containing 5% fetal bovine serum (Biological Industries Ltd., Beit HaEmek, Israel). NIH/3T3 cells were cultured at 37˚C and 5% CO2. Inoculated cell density was 5 × 103 cells/cm2.
Cell counting and viabilityPre-cultured 80-90% confluent NIH/3T3 cells were detached using 0.25% trypsin/1 mM ethylenediaminetetraacetic acid (EDTA) solution (FUJIFILM Wako) for 3 min and seeded onto 60-mm dish and 96-well plate at a cell density of 1 × 105 cells/dish and 1 × 103 cells/well, respectively. After overnight culturing, culture medium was changed with DMEM containing specific chloride salts of alkali metals (Li, Na, K, Rb, and Cs). The cell number and viability of NIH/3T3 cells were measured with TC20TM Automated Cell Counter (Bio-Rad Laboratories, Inc., Hercules, CA, USA) every 24-h using 0.4% trypan blue. The lactate dehydrogenase (LDH) activity and relative NIH/3T3 cell growth were measured at 72 h using a Cytotoxicity LDH Assay Kit-WST and a Cell Counting Kit (Dojindo Laboratories, Kumamoto, Japan), according to the manufacturer’s instructions.
Scratch assayPre-cultured 80-90% confluent NIH/3T3 cells were detached using 0.25% trypsin/1 mM EDTA solution for 3 min and seeded onto a 24-well plate at a cell density of 4 × 105 cells/well. The cells formed a monolayer after overnight culture. The monolayered cells were stained with 1 µg/mL calcein-acetoxymethyl ester (Dojindo) and kept in an incubator for 40-60 min. Wounds were scratched by a 200 µL micropipette tip. Fluorescence images were taken by the BZ-9000 fluorescence microscope system (Keyence, Osaka, Japan). Culture medium was changed with DMEM containing 0, 1, 3, and 10 mM of NaCl and CsCl. Images of gap closure were taken after 24-h incubation. The cell area was measured using ImageJ software (https://imagej.nih.gov/ij/). The percentage of wound closure of NIH/3T3 cells was calculated by the following equation; wound closure (%) = ((initial wound area − final wound area) / initial wound area) × 100.
Statistical analysisData were expressed as mean ± standard deviation. The statistical analysis was performed with one-way analysis of variance by Dunnett’s method using IBM SPSS statistical software (SPSS Inc, Chicago, IL, USA). Statistical significance was set as p < 0.05.
We investigated whether Cs affected cell proliferation in the NIH/3T3 cells. Effects of alkali metal elements on NIH/3T3 cell growth were assessed by WST assay (Figure 1). CsCl treatment decreased NIH/3T3 cell growth in a dose-dependent manner, and its cell growth inhibiting effect was highest among the examined alkali metal elements. The data were fitted using the equation: Y = A / [1 + (X-Cl / EC50)], where Y is the relative cell growth ratio (%), A is the control cell ratio (without alkali metal element addition), X-Cl is the alkali metal chloride salt concentration in medium (mM), EC50 is the effective concentration (mM). The estimated EC50 values of Li, Na, K, Rb, and Cs were 15.5 ± 4.5 mM, 85.7 ± 31.9 mM, 81.0 ± 23.4 mM, 13.4 ± 3.0 mM, and 2.2 ± 0.3 mM, respectively. The results showed that Cs had the most effect on NIH/3T3 cell growth among the alkali metal elements.
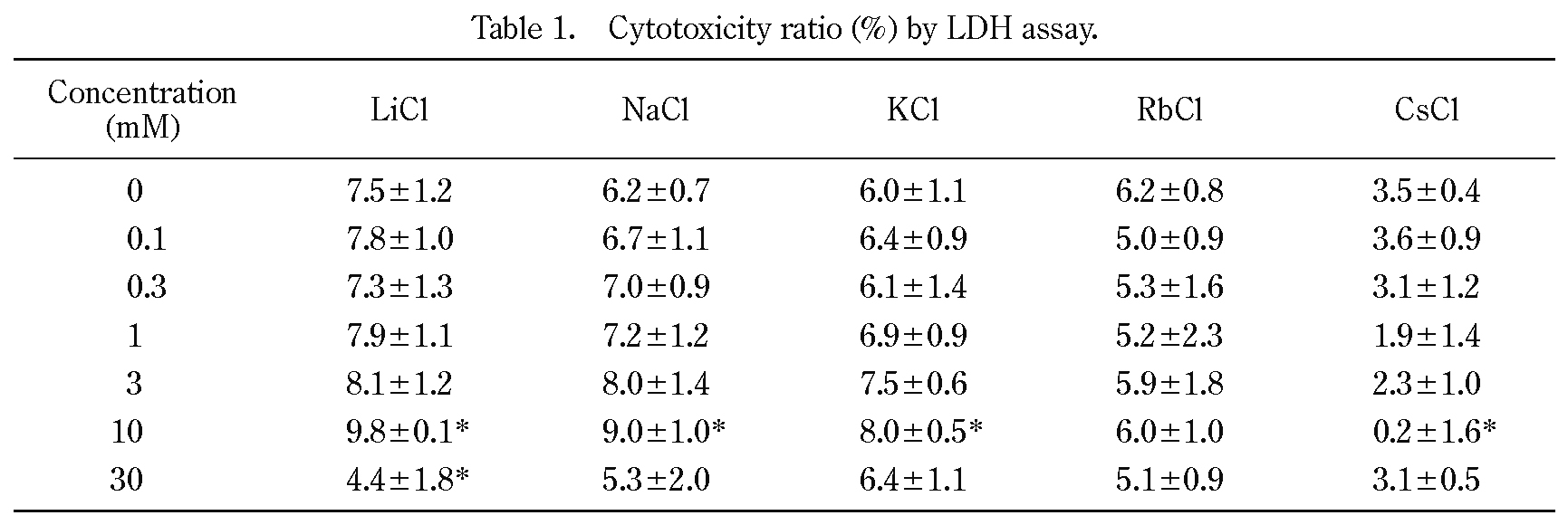
Effects of alkali metal elements on cytotoxicity were assessed by leakage of intracellular LDH activity in NIH/3T3 cells (Table 1). The LDH activity in the extracellular media indicates plasma membrane integrity. The cytotoxicity was low (< 10% of control) and was not enhanced by treatment using 0.03-30 mM of any alkali metal elements. The extracellular LDH activity and concomitant plasma membrane damage were not affected by treatment with any alkali metal elements, in doses up to 30 mM.
The cell numbers were counted after the addition of 0, 1, 3, and 10 mM of NaCl or CsCl to the medium and their viabilities and respective specific growth rates were calculated (Figure 2, Figure 3). The specific growth rates after application of 0, 1, 3, 10 mM NaCl were 0.039 ± 0.004 h−1, 0.038 ± 0.004 h−1, 0.037 ± 0.005 h−1, and 0.037 ± 0.003 h−1, respectively. The specific growth rates were not significantly changed by any concentration of NaCl. On the other hand, after the applying the same concentration series of 1, 3, 10 mM CsCl, the specific growth rates were 0.032 ± 0.006 h−1, 0.026 ± 0.003 h−1, and 0.011 ± 0.003 h−1, respectively (n = 5). The specific growth rate was significantly decreased after adding 3 mM and 10 mM CsCl in comparison with the control (0 mM NaCl equally 0 mM CsCl). In the case of 10 mM CsCl treatment, the specific growth rate was about 0.3-fold in comparison with the control (Figure 3). Although cell proliferation was suppressed by concentrations of 1 mM CsCl and higher, cell viability was not remarkably decreased in the same conditions. The relationship between cell viability and cell proliferation was in good agreement with previously reported data regarding HeLa cells9).
The effects of NaCl and CsCl on NIH/3T3 cell migration were evaluated (Figure 4). The wound closures of NIH/3T3 cells after 24-h incubation with 0, 1, 3, and 10 mM NaCl were 74.1 ± 7.8%, 75.2 ± 7.0%, 77.5 ± 4.8%, and 77.9 ± 4.5% (n = 5), respectively (Figure 5). On the other hand, the wound closures of NIH/3T3 cells after 24-h incubation with 0, 1, 3, and 10 mM CsCl were 75.2 ± 4.6%, 69.6 ± 6.6%, 57.3 ± 5.1, and 36.6 ± 3.6% (n = 5), respectively (Figure 5). The wound closure of NIH/3T3 cells significantly decreased after 3 and 10 mM CsCl treatment in a dose-dependent manner; however, there were no significant differences regarding the wound closure after NaCl treatment.

Effects of alkali metal elements on NIH/3T3 cell growth.
Cells were cultured in the presence of 0, 0.03, 0.1, 0.3, 1, 3, 10, and 30 mM of alkali metal chloride salts (n = 3) for 3 days at 37˚C with 5% CO2. The data were fitted using the equation described in the text. The data are shown as the means of three measurements and error bars indicate the standard deviations. Significant differences in comparison with the control (0 mM) and each treatment are indicated by asterisks (p < 0.01 using Dunnett’s test).
The data are presented as the means ± standard deviations (n = 4). Significant differences between the control (0 mM) and each treatment are indicated by asterisks (p < 0.05 using Dunnett's test).

Effects of cesium on NIH/3T3 cell viability.
Cells were cultured in the presence of 0, 1, 3, and 10 mM of NaCl and CsCl (n = 6) for 3 days at 37˚C with 5% CO2. Cell viability was estimated by using the trypan blue dye exclusion assay. The data are shown as the means of six measurements and error bars indicate the standard deviations. There were no significant differences between the controls (0 mM) and each treatment by Dunnett’s test (p < 0.05).

NIH/3T3 cell proliferation was suppressed by cesium.
(A) Cells were cultured in the presence of indicated NaCl and CsCl in the media for 3 days. The data represent the means of replicate measurements and error bars indicate the standard deviations (NaCl, n = 5 ; CsCl, n = 5). Cell proliferation was fitted to the equation : N = N0 × eμT, where N is the cell number, N0 is the initial cell number, e is the Napier’s constant, μ is the specific growth rate (h−1), and T is the incubation time (h). (B) The specific growth rate. The data are presented as the means of five measurements, and error bars indicate the standard deviations. Significant differences between the controls (0 mM) and each treatment are indicated by asterisks (p < 0.05 using Dunnett's test).
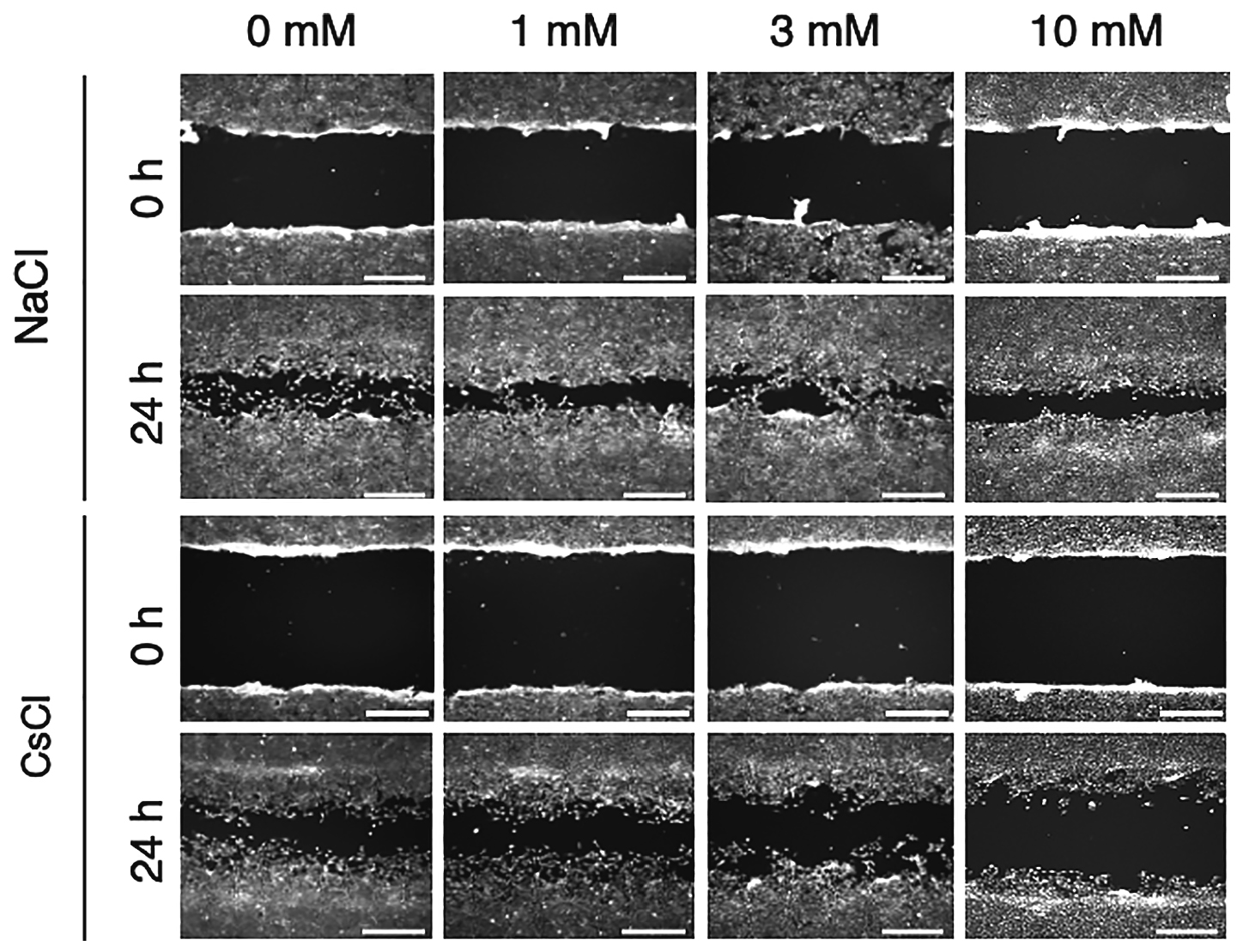
NIH/3T3 cell migration was suppressed by cesium.
Fibroblast cells were grown to confluency, then scratch wound was made. The cells were cultured in DMEM with indicated concentrations of NaCl and CsCl for 24-h. Scale bar indicates 0.5 mm.

Percentage of wound closure in Cs treatment decreased in a dose-dependent manner.
Percentage of wound closure was calculated using the following equation : wound closure (%) = ((initial wound area − final wound area) / initial wound area) × 100. Data are shown the means ± standard deviations (n = 6). Asterisk indicated significant difference between control and each treatment by Dunnett's test (p < 0.05).
We previously reported that Cs suppressed cell proliferation in HeLa cells, the human cervical cancer cell line, in a dose-dependent manner9). In the present study, we evaluated the effects of Cs on cell proliferation and migration in murine embryotic fibroblast NIH/3T3 cells. The effect of alkali metal elements on cell growth in NIH/3T3 cells (Figure 1) indicated that Cs has the highest inhibiting effect among all the examined alkali metal elements. We evaluated NIH/3T3 cell growth by using WST assay, i.e., the cell growth indicated by intracellular reduction activity caused mitochondria reduction activity. On the other hand, cytotoxicity was evaluated by LDH assay, i.e., the increase in the extracellular LDH activity depends on plasma membrane leakage; therefore, it indicated cytomembrane integrity. Cytotoxicity of the all tested elements in dose up to 30 mM was less than 10% compared with controls without additional elements. These results suggested that cell growth suppression by Cs did not induce cell death but decreased net cell numbers. This finding was further supported by the comparison of a specific growth rate (Figure 3). The specific growth rate was not changed by Na treatment; however, it decreased in a dose-dependent manner in Cs treatment.
Fibroblasts play a crucial role in wound healing processes, and become activated to migrate into the damaged tissue. Excessive deposition of ECM is characteristic of fibrotic lesion, and it is known that fibroblasts are a source of ECM in fibrotic lesions. Therefore, fibroblasts are focused on as a target to prevent fibrosis. The migration activity of NIH/3T3 fibroblast cells was assessed by scratch assay in the presence and in the absence of Cs (Figure 4, Figure 5). The wound closure of NIH/3T3 cells significantly decreased after 3 and 10 mM CsCl treatment compared with the control cells. Especially, after 10 mM CsCl treatment, the wound closure of NIH/3T3 cells decreased to approximately 50%. The results showed good correlation between wound closure and specific growth rate. It was anticipated that the decreases in growth rate led to decreases in cell number and cell mobility; therefore, it was thought that the decreases in the NIH/3T3 cells wound closure were not directly related to the NIH/3T3 cells migration activity. Thus, further investigation into whether single cell mobility is inhibited by Cs is necessary.
The authors declare no conflicts of interest associated with this manuscript.