2025 Volume 100 Article ID: 24-00079
2025 Volume 100 Article ID: 24-00079
To explore the oncogenic mechanism of FOXM1 in the tumor microenvironment (TME) regarding triple-negative breast cancer (TNBC) promotion, the mRNA and protein levels of target genes in TNBC cells and their exosomes were detected by RT-qPCR and western blot. A co-culture model of TNBC cells and THP-1/M0 macrophages was established to detect the impact of co-culture on FOXM1 expression and the direction of macrophage polarization. A bioinformatics website was used to predict FOXM1 binding sites in the IDO1 promoter, which were further validated using dual-luciferase reporter and chromatin immunoprecipitation assays. Next, after erastin-induced ferroptosis, we conducted cell viability assays, apoptosis assays and other experiments to investigate whether the FOXM1/IDO1 axis regulates M2 macrophage polarization through ferroptosis. We found that FOXM1 was abundant in exosomes derived from TNBC cells, and that TNBC cells upregulated FOXM1 expression in THP-1 cells through exosomes to promote M2 macrophage polarization. Furthermore, FOXM1 upregulated IDO1 in M2-type tumor-associated macrophages (TAMs) by stimulating its transcription. Finally, FOXM1/IDO1 inhibited ferroptosis, promoting M2 macrophage polarization, thereby advancing TNBC progression. In conclusion, FOXM1 carried by TNBC cell-derived exosomes activated IDO1 transcription in TAMs to inhibit ferroptosis, promoting M2 polarization of TAMs and exerting carcinogenic effects.
Triple-negative breast cancer (TNBC) is a subtype of breast cancer and a common aggressive malignant tumor. Its main treatment modalities include chemotherapy, immunotherapy and targeted therapy. TNBC has a poor prognosis compared to other breast cancer subtypes. Despite the continuous improvement of treatment strategies for TNBC, they still have limitations (Won and Spruck, 2020; Obidiro et al., 2023). This necessitates ongoing research into TNBC.
The transcription factor FOXM1 is closely associated with the development and malignant phenotype of TNBC, with numerous studies reporting its oncogenic role in TNBC (Wang et al., 2021b; Zhang et al., 2021). Exosomes play a crucial role in intercellular communication by transferring RNA, proteins, DNA, lipids and metabolites to target cells, thus altering their fate. Exosomes derived from cancer cells impact drug resistance, progression and metastasis (Zhang and Yu, 2019; Kalluri and LeBleu, 2020). Tumor-associated macrophages (TAMs) are key components of the tumor microenvironment (TME) and can be classified as M1 and M2 macrophages, of which M2-type macrophages support tumor development. Notably, exosomes can also act as inducers of M2-like TAM polarization to accelerate malignant tumor progression (Wang et al., 2021a), and agents that inhibit M2 macrophage polarization are now clinically available (Lin et al., 2019). However, whether FOXM1 can exert its oncogenic effects in the TME via the exosome pathway remains unknown, and we focus here on exploring this topic.
High expression of IDO1 is associated with tumor invasiveness and adverse patient reactions. It promotes the conversion of tryptophan (TRP) to kynurenine (KYN), which induces T cell dysfunction and immune suppression in the TME, facilitating tumor progression (Shi et al., 2022). IDO1 also reportedly promotes M2 polarization and oncogenic effects (Chen et al., 2023). Downstream metabolites of KYN play an anti-ferroptosis role through reactive oxygen species (ROS) clearance and activation of the NRF2 signaling pathway, thereby protecting cancer cells (Fiore et al., 2022). This suggested the importance of including IDO1 in our research scope.
In this study, we initially verified that TNBC cell-derived exosomes are rich in the FOXM1 protein. Additionally, overexpressing FOXM1 in macrophages induced their M2 polarization, indicating that TNBC cells convey FOXM1 via exosomes to promote the M2 polarization of TAMs. Finally, we investigated the positive regulatory effect of FOXM1 on IDO1 expression and the regulatory mechanism of the FOXM1/IDO1 axis.
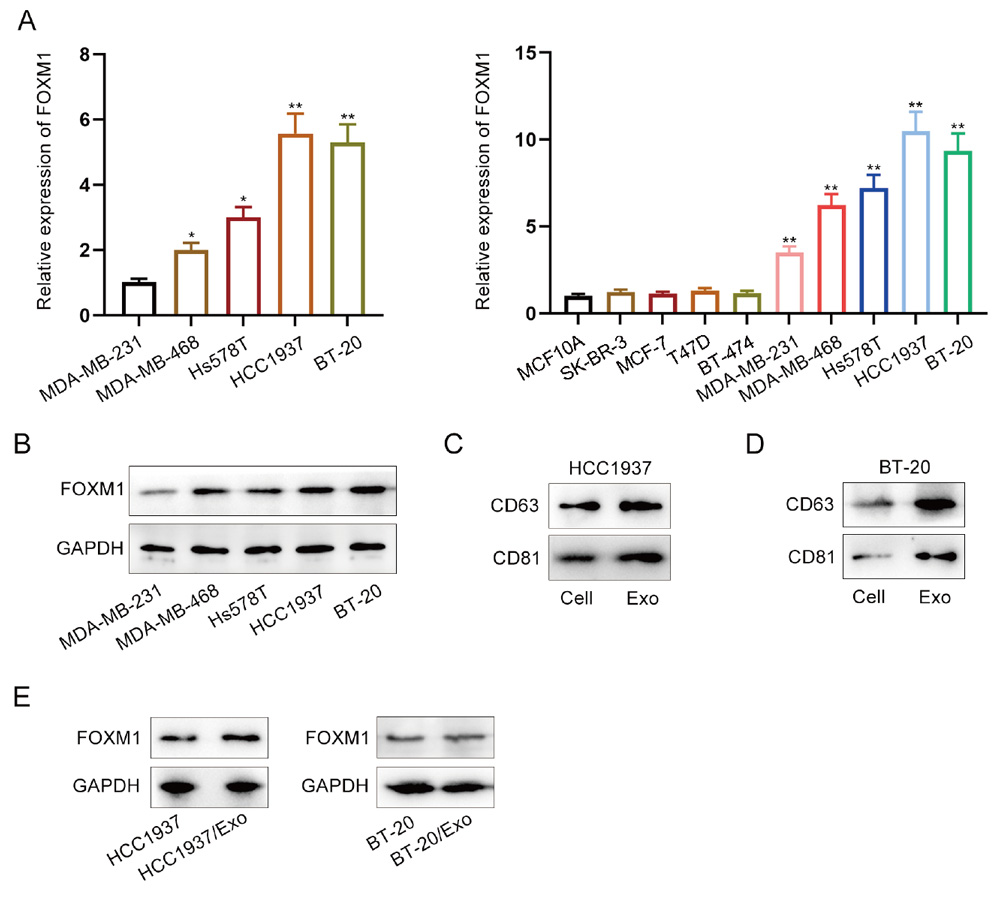
First, we examined the expression of FOXM1. To assess FOXM1 expression levels in TNBC cells (MDA-MB-231, MDA-MB-468, Hs578T, HCC1937 and BT-20), we performed RT-qPCR in normal breast epithelial cells (MCF10A) and cells of different breast cancer subtypes (SK-BR-3, MCF-7, T47D and BT-474) (Fig. 1A). The results indicated that FOXM1 expression was significantly higher in breast cancer cells compared to normal breast epithelial cells (MCF10A), with the highest levels observed in HCC1937 and BT-20 among the TNBC cell lines, prompting further experiments with these two cell lines. Western blot results also showed a similar trend (Fig. 1B). Subsequently, we identified exosomes derived from TNBC cells via western blot, as the exosomes extracted by centrifugation were enriched with the characteristic marker molecules CD63 and CD81 (Fig. 1C, 1D). This confirmed the presence of exosomes from TNBC cells and their successful extraction. Western blot analysis also revealed high levels of FOXM1 in these TNBC cell-derived exosomes (Fig. 1E). This suggests that FOXM1 utilizes TNBC-derived exosomes as carriers to exert its effects in the TME.
TNBC cells upregulate FOXM1 expression in THP-1 cells via exosomes, promoting M2 macrophage polarization
We treated THP-1/M0 cells with exosomes derived from TNBC cells (Fig. 2A). Observing the images obtained from the exosome tracking experiment, we found that these exosomes were taken up by THP-1/M0 cells (Fig. 2B). Meanwhile, RT-qPCR and western blot results showed that the level of FOXM1 mRNA in THP-1/M0 cells was not significantly different after exosome treatment, but the protein level increased markedly (Fig. 2C, 2D). This supports our idea that TNBC cells use exosomes to deliver FOXM1 to play a role in the TME. However, whether exosomal FOXM1 promotes M2 macrophage polarization was unclear and required further experimentation. Changes in the expression of M2 macrophage polarization markers were detected by RT-qPCR, and we found that the exosome treatment increased the expression of CD206 and Arg-1, which promoted M2 polarization (Fig. 2E). In addition, the expression of M2 macrophage polarization markers was significantly elevated after FOXM1 overexpression treatment in THP-1/M0 cells (Fig. 2F, 2G). This strengthens the argument that FOXM1 in exosomes derived from TNBC cells promotes M2 macrophage polarization.

FOXM1 upregulates IDO1 expression in M2 polarized TAMs and promotes M2 macrophage polarization
To determine whether FOXM1 affects M2 polarization in macrophages by regulating IDO1, we performed the following experiments. From Figure 3A and 3B, we learned that TNBC cell co-culture and overexpression of FOXM1 in THP-1/M0 macrophages promote IDO1 expression, which suggests that exosomal FOXM1 positively regulates IDO1 in TAMs. Next, we performed rescue experiments to verify that FOXM1 affects M2 polarization of macrophages via IDO1, and found that the promotion of CD206 and Arg-1 by overexpression of FOXM1 in THP/M0 macrophages was inhibited by knockdown of IDO1 (Fig. 3C, 3D). We then proceeded to investigate the specific regulatory mechanism of FOXM1 on IDO1. Since FOXM1 is a transcription factor, we predicted that it could bind to the promoter region of IDO1 to regulate IDO1 expression. Using the UCSC website, we found that the IDO1 promoter has four FOXM1-binding sites (Fig. 3E). We found binding in both THP-/M0 cells and THP-1/M2 cells by ChIP assays, but the binding level of site 3 was higher in THP-1/M2 macrophages (Fig. 3F). Finally, we mutated site 3 and observed changes in IDO1 promoter activity using a dual-luciferase reporter assay. Analysis of the results showed that overexpression of FOXM1 significantly upregulated luciferase activity in the wild-type promoter (WT) group, but had no significant effect on the mutated promoter (MUT) group (Fig. 3G). This indicates that FOXM1 mainly binds to site 3 in the IDO1 promoter to regulate IDO1 transcription. Taken together, our findings indicate that FOXM1 promotes M2 polarization of macrophages by upregulating IDO1 expression through binding to the IDO1 promoter.

The FOXM1/IDO1 axis promotes M2 polarization of TAMs by inhibiting ferroptosis
Ferroptosis is a recently identified form of programmed cell death distinct from apoptosis, autophagy and necroptosis, and is characterized by the accumulation of iron and lipid peroxides (Li et al., 2020). Moreover, ferroptosis has been reported to play an important role in the diagnosis, progression and treatment of TNBC (Li et al., 2023). Additionally, IDO1 is closely associated with ferroptosis and can inhibit this process (Zeitler and Murray, 2023). Therefore, we investigated whether the FOXM1/IDO1 axis promotes M2 polarization in TAMs by inhibiting ferroptosis. After we induced ferroptosis using erastin, we detected changes in CD206 and Arg-1 expression in THP-1/M2 macrophages using RT-qPCR. The results showed that as the concentration of erastin increases, the expression levels of CD206 and Arg-1 decrease progressively (Fig. 4A). Based on this, we conclude that inhibition of ferroptosis promotes M2 polarization of macrophages. Subsequently, we performed CCK-8 and TUNEL assays in erastin-treated THP-1/M0 cells (Fig. 4B, 4C). We found that knockdown of IDO1 reversed the promotion of cell viability and the inhibition of apoptosis by overexpression of FOXM1. Finally, we found that knockdown of IDO1 also reversed the inhibitory effect of overexpression of FOXM1 on ROS levels and malondialdehyde (MDA, an end product of lipid peroxidation) content in erastin-treated THP-1/M0 (Fig. 4D, 4E). Moreover, RT-qPCR results showed that knockdown of IDO1 reversed the promotional effect of overexpression of FOXM1 on ferroptosis suppressor genes (Fig. 4F). In summary, the FOXM1/IDO1 axis promotes M2 polarization in macrophages by inhibiting ferroptosis.

TNBC is a subtype of breast cancer characterized by its high metastatic potential, and research into therapeutic treatments for this malignancy remains a major challenge. Fortunately, through sustained research, numerous promising therapeutic targets for TNBC have been identified, such as ADAM8, HER3 and the TME (Deepak et al., 2020; Alawak et al., 2021; Lyu et al., 2023). Furthermore, it has been demonstrated that FOXM1 is upregulated in TNBC and represents an effective therapeutic target: inhibition of its activity can suppress the progression and metastasis of TNBC (Dey et al., 2020). Consistent with these findings, our experiments also revealed high expression of FOXM1 in TNBC cells.
Regulatory mechanisms of FOXM1 in other cancers have been explored. For instance, inhibiting FOXM1 enhances immunotherapy efficacy in non-small-cell lung cancer by downregulating PD-L1 and inhibiting cell proliferation (Madhi et al., 2022). FOXM1 promotes pancreatic cancer progression by upregulating miR-552 to target tumor suppressors (DACH1, PCDH10, SMAD4) (Wang et al., 2021c). In TNBC, FOXM1 has been revealed to induce KIF23 expression, promoting EMT and activating the Wnt/β-catenin pathway to drive TNBC progression (Li et al., 2022). Unlike studies on FOXM1 regulation in these cancers, we investigated whether FOXM1 utilizes exosomes for transmission to influence TAM polarization. We found a high abundance of FOXM1 in TNBC cell-derived exosomes. By establishing a co-culture model of TNBC cells and macrophages, we confirmed that TNBC cells transmit FOXM1 via exosomes to promote M2 polarization of macrophages.
FOXM1 can act in cancer through transcriptional regulation, post-transcriptional regulation and post-translational modifications (Liao et al., 2018). In our study, we explored the downstream regulatory mechanisms of FOXM1. We found that FOXM1 supports TNBC development through transcriptional regulation. Based on the experimental results, we found that FOXM1 upregulates IDO1 expression by binding to the IDO1 promoter, which in turn promotes M2 polarization of macrophages by inhibiting ferroptosis. It is noteworthy that IDO1 underwent regulation by exosomal FOXM1 in our study, whereas it may be regulated by other genes in other cancers. For example, IDO1 is negatively regulated by miRNAs in human colon cancer (Lou et al., 2019).
Although our experiments indicate that the FOXM1/IDO1 axis is a promising potential target for treating TNBC, there are some limitations to this study. We have only validated the role of the FOXM1/IDO1 axis in TNBC at the cellular level, and lack in vivo experimental data and direct clinical evidence. Therefore, future research will focus on in vivo and clinical studies.
TNBC cell lines (MDA-MB-231, MDA-MB-468, Hs578T, HCC1937, BT-20) and the human THP-1 cell line were obtained from ATCC (Manassas, VA, USA). Except for HCC1937 and THP-1, which were cultured in RPMI 1640 medium, the other ATCC cell lines were cultured in DMEM. The media of these cells were supplemented with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin (P/S). The normal mammary epithelial cell line MCF10A and other breast cancer subtype cell lines (SK-BR-3, MCF-7, T47D and BT-474) were purchased from Pricella (Wuhan, China). MCF10A cells were cultured in DMEM/F12 supplemented with 5% horse serum, 20 ng/ml epidermal growth factor, 0.5 μg/ml hydrocortisone, 10 μg/ml insulin, 1% non-essential amino acids (NEAA) and 1% P/S. SK-BR-3 cells were cultured in McCoy’s 5A medium supplemented with 10% FBS and 1% P/S. MCF7 cells were cultured in MEM (with NEAA) supplemented with 10 μg/ml insulin, 10% FBS and 1% P/S. T47D cells were cultured in RPMI 1640 medium supplemented with 10 μg/ml insulin, 10% FBS and 1% P/S. The culture conditions for BT-474 cells were similar to those for T47D cells, with the additional supplementation of 2 mM L-glutamine in the medium. Cells were maintained at 37 °C in a 5% CO2 humidified atmosphere. To obtain THP-1/M0 macrophages, THP-1 cells were treated with 100 ng/ml phorbol 12-myristate 13-acetate for 24 h. Polarization of THP-1/M0 macrophages to THP-1/M2 macrophages was induced by 20 ng/ml IL-4 for 24 h. For co-culture models, HCC1937 and BT-20 cells were co-cultured with THP-1/M0 macrophages. Erastin was used to induce ferroptosis in THP-1/M2 macrophages.
Vector construction and transfectionsiRNA targeting IDO1 (si-IDO1) and its negative control siRNA (si-NC), along with the FOXM1 overexpression construct and the blank vector as a control, were supplied by Thermo Fisher Scientific (Waltham, MA, USA) and Ribobio (Guangzhou, China). THP-1/M0 cells were transfected with these vectors using Lipofectamine 3000 reagent (Thermo Fisher Scientific).
Reverse transcription quantitative real-time PCR (RT-qPCR)Total RNA was obtained from the cells using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) and then reverse transcribed using the RevertAid RT Reverse Transcription Kit (Thermo Fisher Scientific). AceQ qPCR SYBR Green Master Mix (Vazyme, Nanjing, China) was used for RT-qPCR. All the above reagents and kits were used according to the instructions provided by the manufacturer. The qPCR procedure began with an initial denaturation at 95℃ for 2 min. This was followed by 40 cycles of denaturation at 95℃ for 15 s and annealing/extension at 60℃ for 1 min. GAPDH was used as an internal reference in this experiment, and the 2-ΔΔCT method was utilized to process the data. Primer sequences are as follows:
Total proteins from HCC1937, BT-20 and THP-1/M0 cells were obtained using RIPA lysis buffer (Beyotime, Shanghai, China). After protein separation on SDS-PAGE, they were transferred to PVDF membranes (Millipore, Billerica, MA, USA), which were then blocked with 5% skimmed milk for 1 h. Incubation with primary antibodies at 4 °C overnight allowed binding to target proteins. The primary antibodies used were anti-FOXM1 (Abcam, Cambridge, MA, USA, ab207298), anti-IDO1 (Abcam, ab211017), anti-CD206 (Abcam, ab125028), anti-Arg-1 (Abcam, ab133543) and anti-GAPDH (Abcam, ab128915). After TBST washing, the membranes were incubated with an HRP-conjugated secondary antibody at room temperature for 1 h; the secondary antibody was goat anti-rabbit IgG H&L (HRP) (Abcam, ab97051). Finally, a chemiluminescence reaction using ECL substrate (Millipore) visualized protein bands. GAPDH served as an internal control. To extract exosomes, the cell culture medium was subjected to sequential centrifugation at 300 × g for 10 min, followed by 2,000 × g for 15 min, and then at 10,000 × g for 30 min. After filtering the supernatant, exosomes were isolated by ultracentrifugation at 100,000 × g for 60 min. For exosome identification, total proteins extracted after centrifugation were obtained using RIPA lysis buffer (Beyotime), followed by detection of CD63 and CD81 protein levels using anti-CD63 (Abcam, ab134045) and anti-CD81 (Abcam, ab155760) antibodies.
Exosome tracking experimentHCC1937 and BT-20 exosomes were labeled with the fluorescent dye PKH26 (Sigma-Aldrich, St. Louis, MO, USA) to appear red. Labeled exosomes were co-cultured with cells for 24 h. After fixation with formaldehyde, fluorescence intensity and exosome distribution were observed using a fluorescence microscope.
Dual-luciferase reporter assayWild-type and mutant luciferase reporter gene vectors containing potential binding sites in the IDO1 promoter region were provided by Sangon (Shanghai, China). Specifically, the WT sequence of site 3 on the IDO1 promoter (chr8:39913359-39913566) and the MUT sequence, which has a mutation in the binding site GGAAACA, were cloned into the pGL3 basic vector. After transfection of THP-1/M0 macrophages with these vectors, luciferase activity was measured using the dual-luciferase reporter assay kit (Promega, Madison, WI, USA).
Chromatin immunoprecipitation (ChIP) assayThe ChIP assay was performed according to a previous report (Lv et al., 2023) using the SimpleChIP Plus Enzymatic Chromatin IP Kit (Magnetic Beads) from Cell Signaling Technology (Danvers, MA, USA). After obtaining the immunoprecipitates, enrichment levels were detected by PCR. The primers for the IDO1 promoter were as follows:
To assess ferroptosis in THP-1/M0 macrophages under different treatments, assay kits for detecting ROS levels (Mlbio, Shanghai, China) and MDA content (Solarbio, Beijing, China) were used in accordance with the manufacturers’ instructions.
CCK-8 assayCell viability of THP-1/M0 macrophages was assayed using the Cell Counting Kit-8 (Beyotime) according to the instructions provided by the manufacturer. Briefly, 2000 cells in 100 µl were plated per well of a 96-well plate for incubation, and 10 µl CCK-8 solution was added to each well for treatment. Finally, absorbance was measured at 450 nm using a microplate reader at appropriate time points (0 h, 2 h, 4 h and 6 h).
Terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) assayTHP-1/M0 macrophages were transfected with empty vector or FOXM1-, si-NC- and si-IDO1-containing vectors. Apoptosis was then predicted using the Click-iT Plus TUNEL assay kit (Thermo Fisher Scientific) according to the manufacturer’s instructions. DAPI stained the nuclei blue. Experimental images were obtained using fluorescence microscopy, and the images were merged and analyzed using ImageJ.
Bioinformatic analysisThe UCSC database (https://genome.ucsc.edu/) was used to predict potential binding sites for FOXM1 in the IDO1 promoter.
Statistical analysisAll experiments were performed more than three times individually. All results are presented as means with standard deviations. Statistical significance was determined by the Student’s t test or ANOVA. GraphPad Prism 7 or SPSS 21.0 (IBM, Armonk, NY, USA) was also used to analyze the data. A P-value of less than 0.05 was regarded as statistically significant; * indicates P < 0.05, and ** indicates P < 0.01.
Funding: This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
Conflicts of interest: None.
Availability of data and materials: All data generated or analyzed during this study are included in this article. Further enquiries can be directed to the corresponding author.