ABSTRACT
YchF is a universally conserved unconventional G protein. It is known to be involved in the translation of leaderless mRNA. However, leaderless mRNA is rare in Escherichia coli under normal culture conditions, so we analyzed E. coli YchF to clarify its function in vivo. First, bioinformatics analysis was performed, and then the growth and survival of ychF mutants were investigated. The results suggest that the functional domains and important amino acid residues of YchF are conserved. We next found that the ychF mutants exhibited delayed re-growth in late stationary phase in the presence of oxidative stress. Moreover, the growth inhibition by catalase overexpression was suggested to be caused by oxidase activity. We found that the E. coli ychF mutants exhibited reduced growth in early stationary phase that was associated with a decreased level of ribosomal 70S subunit. In the ychF mutants, we also found that overproduction of the ribosomal protein S18 inhibited growth, which was further suppressed by overproduction of S11. YchF of E. coli is involved in the regulation of ribosomal 70S levels possibly through interaction with ribosomal proteins S18 and S11 as well as IF-3, suggesting that YchF is important for growth and survival in the early and late stationary phase of growth.
INTRODUCTION
Our research focus is the identification, functional analysis and generation of genome-reduced Escherichia coli strains (Kotaka et al., 2023). Although the functions of all essential genes in E. coli have been identified experimentally, identification of the minimal genome has not yet been achieved due to the relatively large genome size. The minimal genome of Mycoplasma mycoides has been experimentally identified, and it is composed of 473 genes, including quasi-essential and non-essential genes in addition to the essential genes; however, the functions of as many as 149 of these genes are unclear (Hutchison et al., 2016). Our previous studies elucidated the functions of several essential genes of unknown function in E. coli through genetic analysis (Kurata et al., 2015). In addition, we used genome-reduced E. coli to identify and clarify the functions of non-essential genes important for growth and survival, including novel DNA repair genes (Watanabe et al., 2016), and broadly defined oxidative stress resistance genes (Iwadate et al., 2017). To further understand the minimal genome, we investigated genes of unknown function in the JCVI-syn3.0 synthetic genome, which consists of only 473 artificially designed genes (Hutchison et al., 2016), by analyzing their homologs in E. coli. In this study, mutant strains of one of these genes, ychF, were used to elucidate novel phenotypes related to growth and survival in stationary phase.
YchF is an unconventional G protein belonging to the Obg family of P-loop GTPases that is conserved from E. coli to humans and possesses ATPase activity (Assmann, 2002; Landwehr et al., 2021b; Luo et al., 2022; Lin et al., 2023). Two major functions have been identified for YchF. First, YchF acts as a negative regulator of oxidative stress response pathways. Knockdown of the eukaryotic ychF homolog, Ola1, increases resistance to oxidative stress (Zhang et al., 2009). Overproduction of YchF in Arabidopsis thaliana and E. coli also inhibits antioxidant responses (Wenk et al., 2012; Cheung et al., 2013). In E. coli, YchF interacts with the catalase KatG, and overproduction of YchF reduces catalase activity in cell extracts (Wenk et al., 2012). YchF also interacts with other antioxidant proteins such as thioredoxin A (TrxA) (Hannemann et al., 2016). Escherichia coli YchF functions in a redox-regulated monomer–dimer equilibrium, with the dimer exhibiting low ATPase activity and a marked increase in ATPase activity in the monomer (Hannemann et al., 2016). TrxA maintains redox balance in vivo and interacts directly with YchF to dissociate the dimer. YchF levels decrease under oxidative stress conditions and when cells enter stationary phase. YchF deletion mutants also promote cell survival against multiple stress conditions, including oxidative stress, replication stress and translation stress (Wenk et al., 2012; Hannemann et al., 2016). The second major function identified for YchF is regulation of protein synthesis. YchF interacts with ribosomes (Tomar et al., 2011) and stimulates their ATPase activity (Becker et al., 2012). YchF also interacts with translation initiation factor IF-3, as well as several ribosomal proteins on the surface of small ribosomal subunits (Landwehr et al., 2021a). YchF is thought to be a stress-responsive regulator of leaderless mRNA (lmRNA) since the absence of YchF enhances the anti-binding activity of IF-3 and promotes translation of leaderless mRNA; however, lmRNA is common in bacteria such as Mycobacterium tuberculosis and Deinococcus deserti, but rare in proteobacteria such as E. coli (Landwehr et al., 2021b).
Despite our understanding of the biochemical activities of YchF, little is known about its in vivo functions in E. coli and other bacteria, especially with respect to the phenotypes of deletion mutants. To gain insight into the function of ychF in vivo, bioinformatics and genetic approaches were used to understand the phenotypes of several E. coli ychF mutants.
RESULTS
Bioinformatics analysis of evolutionary conservation
Homologs of ychF are highly conserved across the three domains of life (Landwehr et al., 2021b; Lin et al., 2023). Here, evolutionary conservation of ychF was comprehensively investigated in diverse bacteria living in highly distinct environments. According to annotations in the AnnoTree database, ychF was present in nearly all the analyzed bacterial genomes, including those from diverse phyla (21,952 of 22,271 genomes analyzed, 98.6%) (Fig. 1A). This represents a high rate of conservation comparable with that of essential ribosomal components and translation factors (e.g., IF-1, IF-2). This ychF conservation rate also exceeded those of several minor components of ribosomes and genes related to ribosome biogenesis and translation factors (e.g., small protein S21 and RF-3) (Fig. 1A; Supplementary Fig. S1). The very high conservation rate throughout the bacterial tree of life suggests that ychF has an ancient origin and has essential biological functions that facilitate cell survival in a range of environmental conditions. This is supported by the finding that ychF is essential in some bacterial species according to the database of essential genes (e.g., Staphylococcus aureus N315, Streptococcus pneumoniae, M. pulmonis UAB CTIP, E. coli O157:H7 str. EDL933 and E. coli MG1655) (Luo et al., 2021). Furthermore, ychF is also retained in the minimal genome JCVI syn-3.0 (Hutchison et al., 2016) (Supplementary Table S1). Despite this, single-gene knockout mutants of ychF are viable in E. coli under experimental conditions (Yamazaki et al., 2007; Landwehr et al., 2021a) in which expression of pth is maintained (ychF forms an operon with pth, which encodes an essential peptidyl-tRNA hydrolase) (Cruz-Vera et al., 2002). This suggests that bypass systems compensating for YchF functions have evolved in some contemporary bacteria, including E. coli, or that conditions in which YchF is critical are not encountered under standard experimental culture conditions for E. coli.

Fig. 1. Evolutionary conservation analysis of ychF. (A) Conservation of ychF and ribosome-related and oxidative stress resistance genes. Genes related to ribosome biogenesis are shown in Supplementary Figure S1. Areas marked in yellow indicate presence, and areas marked in purple indicate absence. (B) Universally conserved residues identified by YchF protein alignment. (C) YchF domain architecture in bacteria.
Next, neighboring genes of ychF in bacteria were examined to further elucidate the function and regulation of YchF from its genomic context. Taxon-wide exploration of ychF-neighboring (five genes up- or downstream) and -adjacent (one gene up- or downstream) genes revealed that pth homologs were the most frequently found genes in the proximity of ychF, and were often directly adjacent to ychF (Supplementary Fig. S2A–S2D). The adjacent relationship between ychF and pth was particularly highly conserved in proteobacteria (Supplementary Fig. S2E–S2H). The evolutionary conservation of genomic synteny suggests a functional relationship between pth and ychF, especially in proteobacteria.
To further understand the high levels of evolutionary conservation alongside potential diversities of regulatory mechanisms, the domain architecture evolution of YchF was assessed using 50,806 YchF sequences collected from the Pfam database (proteins with the YchF-GTPase C-terminal domain, PF06071). In general, the domain architecture of YchF was highly conserved in both bacteria and eukaryotes, with a mononucleotide binding domain (PF01926) at the N-terminal and a YchF-GTPase C-terminal domain (46,176/50,806 proteins, 90.8%) (Fig. 1C; Supplementary Fig. S3C and S3D) (Teplyakov et al., 2003). A small proportion (8.6%) of YchF proteins had other additional domains. In bacteria, these additional domains were very rare and were often singletons, possibly indicating annotation errors. By exception, an N-terminal domain of FeoB (the inner membrane component of a ferrous iron uptake system), which specifically binds GTP (Marlovits et al., 2002), was found in several species, mostly Chloroflexota (Supplementary Fig. S3E; Supplementary Fig. S4A). More diverse additional domain repertories of YchF-like proteins were observed in eukaryotes (Supplementary Fig. S3F). For example, PUA (pseudouridine synthase and archaeosine transglycosylase) and Pre-PUA domains were found in several Ascomycota species (Supplementary Fig. S4B). These domains are often found in eukaryotic translation initiation factors (eIF1 and eIF2), and may therefore be involved with the ribosome association of YchF. However, the functions of these eukaryotic YchF-like proteins with additional domains are unknown.
Following on from previous research that reported interactions between YchF and oxidative resistance-related genes (e.g., trxA and katG) in E. coli (Wenk et al., 2012; Hannemann et al., 2016), we compared the distribution of oxidative stress-related genes in bacteria. Thioredoxin 1 (TrxA), a regulator of YchF dimerization, was as highly conserved among diverse bacteria as YchF (Fig. 1A). This raises the possibility that regulation of YchF by thioredoxin may be evolutionarily conserved. Conversely, YchF is also known to bind KatG in E. coli (Hannemann et al., 2016), but KatG did not display the same high level of conservation as YchF and TrxA among bacteria (Fig. 1A). In E. coli, oxidation of six cysteine residues of YchF is important in the thioredoxin-mediated regulation of YchF dimerization (Hannemann et al., 2016). Cysteine residue conservation was examined in multiple sequence alignments of YchF sequences from diverse bacteria, but the six cysteine residues were not universally conserved (Supplementary Fig. S3A). Furthermore, the number of cysteine residues found in YchF was highly diverse, and some YchF proteins had no cysteine residues in the entire protein sequence (Supplementary Fig. S3B). Cysteine residues may therefore not be important for the primary function of YchF. By contrast, conserved G1 ATP-binding motif residues of YchF (P11 and N12 in E. coli YchF) (Wenk et al., 2012) and serine residues (S16 in E. coli YchF) were essential for phosphorylation activity, except where serine was substituted with threonine, another important kinase residue. These residues were highly conserved in YchF from diverse bacteria (Fig. 1B), and may be essential for the major functions of YchF.
Growth defect at the late stationary phase under oxidative stress of the ychF mutants
Next, strains of E. coli were constructed that lacked ychF, or harbored mutations at the conserved phosphorylation-essential serine (S16) or ATP-binding motif (P11 and N12) residues. First, a competition assay was performed on the wild-type and ΔychF strains. Mutant and control strains were incubated overnight at 37 °C until stationary phase and were then combined in a 1:1 ratio. The presence of equal numbers of viable bacteria of each strain was confirmed using a spot test. Mixed cultures were diluted 1/100,000 and incubated at 37 °C with or without menadione. For monoculture, the same operation was performed without mixing. After 5 days, the number of viable bacteria was counted by diluting samples 1/50, 1/2,500, and 1/125,000, spotting onto AM3 plates, and culturing at 37 °C. The results showed that the ratio of the ΔychF strain decreased by day 5 when supplemented with menadione to induce oxidative stress (Fig. 2A). To examine the impact of ychF mutations in more detail, spot tests were used to examine the solo growth of wild-type and ychF mutant strains in the presence or absence of oxidative stress. Growth differences between wild-type and ΔychF were minimal after 1 day of incubation (Fig. 2B), but examination at 5 days revealed substantially slower mutant growth in the presence of menadione (Fig. 2C, upper). The growth delay was significant and reproducible when the cells were incubated on plates but it was hard to quantify. This phenotype was complemented by a miniF-ychF plasmid (mFKm-ychF), indicating that the defect was due to YchF deficiency (Fig. 2C, lower). Additionally, individual growth assays of these mutants showed that ychF (S16A), which mimicked the dephosphorylated state, and ychF (P11A N12A), in which ATPase activity was inhibited, had delayed re-growth, whereas ychF (S16E), which represented a permanently phosphorylated state, did not (Fig. 2D). Taken together, these results suggest that the lower proportion of ΔychF in the competition assay was not due to lower survival, but to a delay in re-growth.

Fig. 2. Competition assay and growth delay in ΔychF strains. (A) Equal amounts of wild-type and ΔlacZ or ΔychF strains were mixed and spotted immediately, or after 5 days, as a 50-fold dilution series; both wild-type and mutant strains grew on the Ap- plate, while only mutant strains grew on the Ap+ plate. (B) Differences in growth rate between wild-type and ΔychF strains spotted as a 50-fold dilution series after 1 day of monoculture. (C) Wild-type and ΔychF strains spotted after 5 days of monoculture (upper), and ΔychF growth after 5 days with empty plasmid (mFKm) or a complementation plasmid (mFKm-ychF) (lower). (D) Altered-residue strains spotted after 5 days of monoculture. The competition assay was confirmed by repeating the experiment eight times.
Growth inhibition by overexpression of KatG in the wild-type strain
YchF was previously reported to inhibit the activity of KatG, but the biological function in vivo underlying this is unclear. Therefore, the effect of overproduction of catalase, as well as two previously reported mutants of katG, on the growth of the wild-type strain was assessed. A plasmid in which the coding region of katG was cloned downstream of the PBAD promoter of the pBAD plasmid was introduced into the wild-type strain and grown in the presence of arabinose: growth was slightly inhibited (Fig. 3). The relationship between the activity of catalase and growth inhibition when catalase was overproduced was examined for KatG (W105F) and KatG (H106Y). KatG (W105F) was reported to lack catalase activity, but BpKatG, the Burkholderia pseudomallei equivalent of KatG (W105F), showed enhanced oxidase activity (Hillar et al., 2000; Singh et al., 2004). KatG (H106Y) similarly lacks catalase activity, but also has reduced oxidase activity. Overproduction of KatG (W105F) resulted in stronger growth inhibition than overexpression of wild-type KatG, but no difference in growth inhibition was noted between wild-type KatG- and KatG (H106Y)-overproducing strains (Fig. 3). These results suggest that excess catalase causes growth inhibition, which may be due to the generation of reactive oxygen species by excess oxidase activity. Therefore, the function of YchF as a negative regulator of catalase is important for growth and survival.

Fig. 3. Overexpression of wild-type katG and altered-residue katG variants in medium containing 0.2% arabinose. Growth of the KatG (W105F)-overexpressing strain was inhibited significantly.
Growth defect at the late log phase under oxidative stress of the ychF mutants
Next, examination of growth during log phase showed slight reductions in growth rate during the late log phase and early stationary phase in the ΔychF strain under standard 30 °C culture conditions (Fig. 4A). A lacZ expression assay showed that protein synthesis was reduced only during the early stationary phase (Fig. 4A). This phenotype was complemented by the miniF-ychF plasmid (mFKm-ychF), indicating that this was due to loss of YchF. Similar results were observed with the PBAD promoter, indicating that the phenotype was not specific to the lacZ promoter (Fig. 4B). Similar reductions in expression were also observed in ychF (S16A) and ychF (P11A N12A), but not in ychF (S16E) (Fig. 4B), indicating that phosphorylation of YchF and loss of ATPase activity are responsible for these phenotypes.

Fig. 4. Translational activity of the ΔychF strain. (A) β-galactosidase activity and growth curve. Raw data are plotted as dots, with bars indicating mean values and lines indicating growth (O.D. 600). (B) Promoter activity under conditions of transiently reduced translational activity. Promoter activity under ychF complementation with the mFKm-ychF plasmid, and in altered-residue variants, was measured and tested statistically (*P < 0.05; N.S., not significant). Bars indicate the mean ± standard deviation.
Growth inhibition by overexpression of rpsR in the ΔychF mutant
To gain insight into the function of ychF, an Hpx (katG- katE- ahpCF-; Park et al., 2005) ΔychF strain was generated and transformed with multi-copy plasmids containing a range of genes, as described in our previous study (Iwamoto et al., 2012). Weak growth inhibition was observed with the multi-copy plasmid containing the rpsF region. To observe growth inhibition under simpler conditions, a multi-copy plasmid containing the rpsF operon was introduced into the ΔychF strain. Growth inhibition was induced markedly under low-salt conditions (LB with 0.05% NaCl) at 30 °C (Fig. 5A). Since the rpsF operon contains four genes, rpsF (small protein S6), priB (primosomal replication protein N), rpsR (small protein S18) and rplI (large subunit L9), individual plasmids were generated using inverse PCR to express single rps genes from the native promoter. Overexpression of rpsR caused significant growth inhibition (Fig. 5B; Supplementary Fig. S5). Although the mechanism underlying this growth inhibition is not yet known, the results indicate that there is a genetic interaction, suggesting a functional link.

Fig. 5. Growth inhibition by overexpression of rpsR, and its suppression by overexpression of rpsK. Strains were incubated at 30 °C in low-salt LB medium (0.05% NaCl). (A) Growth inhibition of the ΔychF strain by overexpression of the rpsF-rplI operon. (B) Growth inhibition analysis showed that rpsR was the causative gene within the operon. This phenotype was complemented by the mFKm-ychF plasmid. (C) Suppression of rpsR-induced growth inhibition by rpsK.
The ribosomal protein S18 constitutes the platform of the ribosome small subunit, and assembly of the proteins at the platform site is performed by assembling the S6:S18 complex followed by assembly of rpsK (S11) (Mizushima and Nomura, 1970). Overexpression of a multi-copy plasmid containing the rpsK gene suppressed growth inhibition by rpsR, suggesting a functional link (Fig. 5C). Next, polysome profiling was examined under rpsR overexpression conditions causing growth inhibition, and a decrease in 70S ribosomes and a slight increase in 30S ribosomes were observed in the ΔychF strain in the early stationary phase (Fig. 6A and 6B). Under low Mg2+ conditions (0.1 mM), when 70S ribosomes dissociate, the 50S and 30S ribosomal profiles were almost identical in wild-type and ΔychF (Fig. 6C and 6D). This suggests that 70S ribosomes dissociate into 30S and 50S ribosomes in the absence of YchF. However, the possibility of polysomes or higher molecular weight aggregates cannot be excluded. No precursors were observed as in the case of abnormal ribosome assembly, and quantitative analysis of the ratio of each ribosomal protein by LC-MS/MS showed no significant differences (Supplementary Fig. S6). These results suggest that ribosomal proteins S18 and S11 are involved in YchF function.

Fig. 6. Polysome profiles of strains overexpressing rpsR. Profiles were obtained from the same sample in buffer with different Mg2+ concentrations. (A) wild-type (wt) / pACYC184-rpsR, 10 mM Mg2+; (B) ΔychF / pACYC184-rpsR, 10 mM Mg2+; (C) wild-type (wt) / pACYC184-rpsR, 0.1 mM Mg2+; (D) ΔychF / pACYC184-rpsR, 0.1 mM Mg2+.
DISCUSSION
Recently, the unconventional G protein YchF was shown to be involved in the translation of leaderless mRNA, but its function in E. coli, where leaderless mRNA is rare, is not well understood. In this study, YchF in E. coli was found to be important for early stationary phase growth and for re-growth in the late stationary phase. Previous research suggests that cells showing the growth advantage in stationary phase (GASP) phenotype emerge in the late stationary phase (Finkel, 2006), especially during the late stationary phase when re-growth involves competition among many bacteria. The importance of YchF in late stationary phase re-growth may explain its universal conservation among a wide variety of bacteria, as this growth is key for development and survival in a competitive natural environment.
The highly conserved domain architecture of YchF suggests that its biochemical functions are also conserved. The conserved serine residue (S16 in E. coli YchF) and G1 ATP-binding motif residues in YchF (P11 and N12 in E. coli YchF), which are essential for phosphorylation activity, are highly conserved in bacterial YchF, suggesting that they are important for conserved biochemical functions. By contrast, regulation of YchF activity by thioredoxin is not conserved, and different regulatory mechanisms occur in different bacteria. In addition, KatG is not highly conserved, and the interaction between YchF and KatG is likely to have emerged only in specific evolutionary lineages.
In this study, we showed that stationary phase growth is affected by deletion and point mutations of ychF in E. coli. The lactose operon was down-regulated in the ΔychF mutant in a promoter-independent manner during the early stationary phase, and the polysome profile of the mutant exhibited a decreased amount of 70S subunit, suggesting a defect in protein synthesis. The decrease in 70S subunits was accompanied by a slight increase in 30S subunits, suggesting a possible defect in the formation of 70S subunits from 30S and 50S subunits or higher molecular weight aggregates including polysomes. No 16S rRNA precursors were observed from fractionated 70S and 30S subunits, and no significant differences in subunit composition were found, indicating no defect in ribosome assembly. This is consistent with a recent report that YchF is not involved in ribosome assembly (Gibbs et al., 2020).
Genetic analysis revealed a functional association of YchF with RpsR (S18) and RpsK (S11). S18 and S11 are both located in the platform of the ribosome where IF-3 binds to the 30S subunit (Dallas and Noller, 2001). A previous study reported that YchF interacts with IF-3 (Landwehr et al., 2021a), and physical interactions with YchF have also been shown for RpsR (S18) and RpsK (S11) (Landwehr et al., 2021a). The facts that YchF forms an operon with pth and that its synteny is highly conserved in proteobacteria suggest a functional link between YchF and Pth, which is important in the translation initiation step. Given these findings, the function of YchF may be to support normal execution of the translation process via IF-3. In addition to its function in inhibiting the formation of the 70S subunit by blocking the binding of the 50S subunit during translation initiation (Antoun et al., 2006), IF-3 also has an important function in the dissociation of the 70S subunit during recycling of stalled ribosomes (Singh et al., 2005, 2008). The importance of spatial and temporal regulation of IF-3 binding to ribosomes has been proposed (Landwehr et al., 2021a). The more direct functional association of YchF with IF-3, and the underlying molecular mechanisms, need to be clarified by future studies.
YchF functions as a negative regulator of catalase. Excessive catalase activity inhibited growth, and experiments with katG mutants suggested that this was due to excessive oxidase activity. It is proposed that excess KatG causes cytotoxicity via hydrogen peroxide and superoxide radicals generated by its own oxidase activity (Hannemann et al., 2016). It is possible that in the absence of oxidative stress, YchF suppresses KatG activity, thereby preventing KatG from generating harmful reactive oxygen species. In this work, we found a phenotype of slower reductions in growth rate during the late log phase and early stationary phase and a phenotype of delayed re-growth in the stationary phase in the presence of oxidative stress in the ychF deletion strain (Fig. 7), which does not indicate that the ychF deletion strain is susceptible to oxidative stress and is not consistent with previously published results (Lin et al., 2023). Future studies should aim to clarify the functional linkage between oxidative stress conditions, inhibition of catalase activity and regulation of protein synthesis.
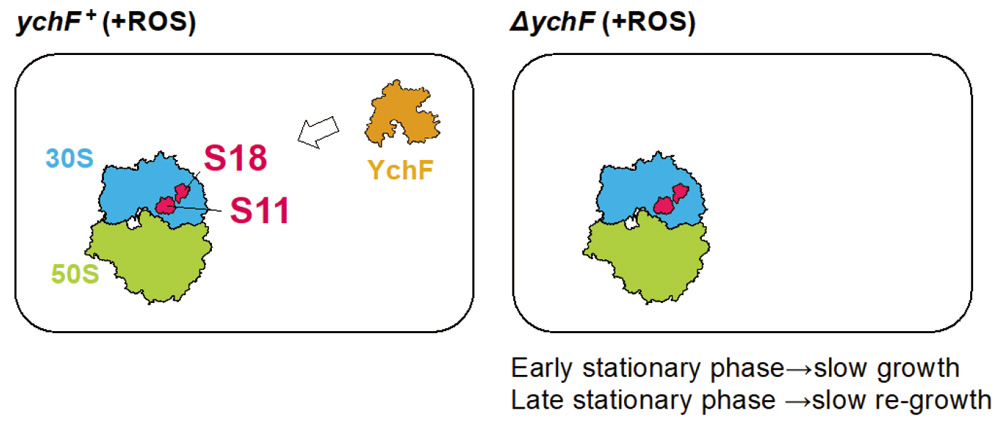
Fig. 7. Involvement of ribosomal proteins S18 and S11 in YchF function, which is important for cell growth at the early stationary phase and re-growth at the late stationary phase.
MATERIALS AND METHODS
Bacterial strains and culture media
All E. coli strains described in this study are derivatives of MG1655. Lists and construction of strains and plasmids used in this work are shown in Supplementary information. Cells were grown in LB medium or Antibiotic Medium 3 (AM3, Becton Dickinson), unless otherwise stated. The approximate composition of AM3 is 1.5 gl-1 beef extract, 1.5 gl-1 yeast extract, 5 gl-1 peptone, 1 gl-1 dextrose, 3.5 gl-1 sodium chloride, 3.68 gl-1 dipotassium phosphate and 1.32 gl-1 monopotassium phosphate. Cells were grown on AM3 or LB plates containing an appropriate drug at the following concentrations: ampicillin, 20 μg/ml; kanamycin, 40 μg/ml; chloramphenicol, 15 μg/ml.
Analysis of evolutionary conservation and essentiality of ychF in bacteria
The presence and absence of genes was assessed using AnnoTree (Mendler et al., 2019), which is a database of the phylogenomic distributions of 28,311 gene/protein families in >27,000 bacterial and >1,500 archaeal genomes based on classification by the genome taxonomy database (GTDB, release 95) (Parks et al., 2022). The presence/absence matrix of Pfam (Mistry et al., 2021) and KEGG Orthology (KO) annotations (Kanehisa and Goto, 2000) in each genome was obtained from https://data.ace.uq.edu.au/public/misc_downloads/annotree/r95/. The distributions of ychF (KEGG KO: K06942), proteins included in ribosomal proteins (KEGG pathway: map03010), ribosome biogenesis (KEGG brite: ko03009), translation factors (KEGG brite: ko03012), trans-translation systems (SmpB: K03664 and ssrA tmRNA: K15035) and oxidative stress resistance genes were examined in E. coli. The distribution of glutathione reductase (gor) (K00383), alkyl hydroperoxide reductase (ahpC) (K03386), Cu-Zn family superoxide dismutase (sodC) (K04565), Fe-Mn family superoxide dismutase (sodA and sodB) (K04564), peroxiredoxin reductase component of alkyl hydroperoxide reductase (ahpF) (K03387), monofunctional catalase (katE) (K03781), bifunctional catalase-peroxidase (katG) (K03782), peptide-methionine (S)-S-oxide (msrA) (K07304), hydrogen peroxide-inducible genes activator (oxyR) (K04761), redox-sensitive transcriptional activator (soxR) (K13639), mar-sox-rob regulon activator (soxS) (K13631), thioredoxin 1 (trxA) (K03671) and thioredoxin reductase (trxB) (K00384) was examined in bacteria. Essentiality data for ychF genes were obtained from DEG15 (Luo et al., 2021) and PEC (Yamazaki et al., 2007).
Domain architecture analysis of bacterial and eukaryotic YchF homologs
For the domain architecture analysis of YchF homologs found in the three domains of life, proteins containing the PF06071 domain (YchF-GTPase_C or DUF933) and its metadata collected in Pfam (Mistry et al., 2021) were downloaded from InterPro (Paysan-Lafosse et al., 2023). The domain architecture of these YchF homologs was analyzed based on a domain search by Pfamscan (Mistry et al., 2007).
Neighbor gene analysis of bacterial ychF
To identify neighbor genes of ychF, ychF was identified in genomes collected in GTDB release 207 (Parks et al., 2022) using the hmmsearch function of HMMER (Eddy, 2011; Finn et al., 2011), based on the profile of PF06071 and according to the gathering threshold in Pfam (Sequence score > 27, Domain score > 27). The five genes up- and downstream of ychF in each genome were collected and functionally annotated by Pfamscan (Mistry et al., 2007) and KofamScan (Aramaki et al., 2020) based on the Pfam domain and KEGG KO, respectively.
Multiple sequence alignment of bacterial YchF
YchF homologs were identified in genomes collected in GTDB release 207 (Parks et al., 2022), as shown below. Proteins were filtered with the presence of the PF01926 domain according to the gathering threshold in Pfam (Sequence score > 21.9, Domain score > 21.9). After filtering, 53,086 YchF proteins with the same domain architecture as E. coli YchF remained. Protein sequences were further clustered based on sequence identity with mmseqs2 with --min-seq-id 0.70 (Steinegger and Söding, 2017). Multiple sequence alignments were calculated using mafft with --large --globalpair options (Katoh et al., 2002) after addition of the E. coli YchF sequence (b1203). The positions of known important residues in E. coli after the multiple sequence alignment were determined by reference to positions in E. coli YchF.
Promoter activity assay
Overnight cultures grown in LB medium were diluted 1/100 in fresh LB (0.5% NaCl). After culture at 30 °C for 2 h, supplements (1 mM IPTG or 0.2% arabinose) were added and culture was continued for another 1 h. β-galactosidase activities were measured three times as previously described (Miller, 1972).
Polysome profiles
Overnight cultures grown in LB medium were diluted 1/100 in fresh LB. After incubation at 37 °C for 2 h, cells were incubated in LB (0.05% NaCl) at 30 °C for another 4 h. Cells were then suspended in buffer A (20 mM Tris–HCl (pH 7.5), 10 mM or 0.1 mM MgCl2, 100 mM NH4Cl and 6 mM β-mercaptoethanol), and lysed by freezing and thawing. After adding deoxycholic acid and incubating on ice for 3 min, cell lysates were centrifuged at 15,000 rpm for 10 min at 4 °C. Absorbance at 260 nm was measured and cells were loaded into a 10–30% sucrose gradient to equalize absorbance at 260 nm. Samples of 350 µl were collected from each fraction and their absorbance was monitored at 260 nm using a UV detector.
LC-MS/MS
Samples were prepared as described previously (Taoka et al., 2014). The resulting peptide samples were analyzed on a nanoscale LC-MS/MS system (Taoka et al., 2009) using a quadrupole-orbitrap hybrid mass spectrometer (Q Exactive, Thermo Fisher Scientific, USA). Peptides were detected in MS mode to select a set of precursor ions for analysis by data-dependent collision-induced dissociation mass spectrometry (MS/MS). Every second, the ten ions with the largest signal intensities were subjected to MS/MS analysis. The MS/MS signals were converted by Proteome Discoverer (Thermo Fisher Scientific), and a search was performed against the UniProt database (version SwissProt_2013_05) using MASCOT version 2.3.2 (Matrix Science, UK) and the following parameters: fixed modification: carbamidomethyl (Cys); variable modifications: oxidation (Met), N-acetylation, pyroglutamine; maximum missed cleavages: 3; peptide mass tolerance: 15 ppm; MS/MS tolerance: 0.8 Da. The criteria for identification were based on vendor definitions (P < 0.05). More stringent criteria were set for protein assignment: > 2 peptides with P < 0.05 were considered to indicate a ‘hit’. Using the hit peptides, quantitation of proteins was performed with Xcalibur 3.0 (Thermo Fisher Scientific).
Statistical analysis
Normality and variance in each dataset were determined a priori using Shapiro–Wilk or Bartlett tests. Welch’s t-test was used to analyze differences between two groups. A Dunnet test was used to analyze differences among more than three groups. P < 0.05 was considered significant.
REFERENCES
- Antoun, A., Pavlov, M. Y., Lovmar, M., and Ehrenberg, M. (2006) How initiation factors tune the rate of initiation of protein synthesis in bacteria. EMBO J. 25, 2539–2550. DOI: 10.1038/sj.emboj.7601140
- Aramaki, T., Blanc-Mathieu, R., Endo, H., Ohkubo, K., Kanehisa, M., Goto, S., and Ogata, H. (2020) KofamKOALA: KEGG Ortholog assignment based on profile HMM and adaptive score threshold. Bioinformatics 36, 2251–2252. DOI: 10.1093/bioinformatics/btz859
- Assmann, S. M. (2002) Heterotrimeric and unconventional GTP binding proteins in plant cell signaling. Plant Cell 14 (Suppl), S355–S373. DOI: 10.1105/tpc.001792
- Becker, M., Gzyl, K. E., Altamirano, A. M., Vuong, A., Urbahn, K., and Wieden, H.-J. (2012) The 70S ribosome modulates the ATPase activity of Escherichia coli YchF. RNA Biol. 9, 1288–1301. DOI: 10.4161/rna.22131
- Cheung, M.-Y., Li, M.-W., Yung, Y.-L., Wen, C.-Q., and Lam, H.-M. (2013) The unconventional P-loop NTPase OsYchF1 and its regulator OsGAP1 play opposite roles in salinity stress tolerance. Plant Cell Environ. 36, 2008–2020. DOI: 10.1111/pce.12108
- Cruz-Vera, L. R., Galindo, J. M., and Guarneros, G. (2002) Transcriptional analysis of the gene encoding peptidyl-tRNA hydrolase in Escherichia coli. Microbiology 148, 3457–3466. DOI: 10.1099/00221287-148-11-3457
- Dallas, A., and Noller, H. F. (2001) Interaction of translation initiation factor 3 with the 30S ribosomal subunit. Mol. Cell 8, 855–864. DOI: 10.1016/S1097-2765(01)00356-2
- Eddy, S. R. (2011) Accelerated profile HMM searches. PLoS Comput. Biol. 7, e1002195. DOI: 10.1371/journal.pcbi.1002195
- Finkel, S. E. (2006) Long-term survival during stationary phase: evolution and the GASP phenotype. Nat. Rev. Microbiol. 4, 113–120. DOI: 10.1038/nrmicro1340
- Finn, R. D., Clements, J., and Eddy, S. R. (2011) HMMER web server: interactive sequence similarity searching. Nucleic Acids Res. 39, W29–W37. DOI: 10.1093/nar/gkr367
- Gibbs, M. R., Moon, K. M., Warner, B. R., Chen, M., Bundschuh, R., Foster, L. J., and Fredrick, K. (2020) Functional analysis of BipA in E. coli reveals the natural plasticity of 50S subunit assembly. J. Mol. Biol. 432, 5259–5272. DOI: 10.1016/j.jmb.2020.07.013
- Hannemann, L., Suppanz, I., Ba, Q., MacInnes, K., Drepper, F., Warscheid, B., and Koch, H.-G. (2016) Redox activation of the universally conserved ATPase YchF by Thioredoxin 1. Antioxid. Redox Signal. 24, 141–156. DOI: 10.1089/ars.2015.6272
- Hillar, A., Peters, B., Pauls, R., Loboda, A., Zhang, H., Mauk, A. G., and Loewen, P. C. (2000) Modulation of the activities of catalase-peroxidase HPI of Escherichia coli by site-directed mutagenesis. Biochemistry 39, 5868–5875. DOI: 10.1021/bi0000059
- Hutchison, C. A., 3rd, Chuang, R.-Y., Noskov, V. N., Assad-Garcia, N., Deerinck, T. J., Ellisman, M. H., Gill, J., Kannan, K., Karas, B. J., Ma, L., et al. (2016) Design and synthesis of a minimal bacterial genome. Science 351, aad6253. DOI: 10.1126/science.aad6253
- Iwadate, Y., Funabasama, N., and Kato, J.-i. (2017) Involvement of formate dehydrogenases in stationary phase oxidative stress tolerance in Escherichia coli. FEMS Microbiol. Lett. 364, fnx193. DOI: 10.1093/femsle/fnx193
- Iwamoto, A., Osawa, A., Kawai, M., Honda, H., Yoshida, S., Furuya, N., and Kato, J.-i. (2012) Mutations in the essential Escherichia coli gene, yqgF, and their effects on transcription. J. Mol. Microbiol. Biotechnol. 22, 17–23. DOI: 10.1159/000336517
- Kanehisa, M., and Goto, S. (2000) KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res. 28, 27–30. DOI: 10.1093/nar/28.1.27
- Katoh, K., Misawa, K., Kuma, K.-i., and Miyata, T. (2002) MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res. 30, 3059–3066. DOI: 10.1093/nar/gkf436
- Kotaka, Y., Hashimoto, M., Lee, K.-i., and Kato, J.-i. (2023) Mutations identified in engineered Escherichia coli with a reduced genome. Front. Microbiol. 14, 1189877. DOI: 10.3389/fmicb.2023.1189877
- Kurata, T., Nakanishi, S., Hashimoto, M., Taoka, M., Yamazaki, Y., Isobe, T., and Kato, J.-i. (2015) Novel essential gene Involved in 16S rRNA processing in Escherichia coli. J. Mol. Biol. 427, 955–965. DOI: 10.1016/j.jmb.2014.12.0133
- Landwehr, V., Milanov, M., Angebauer, L., Hong, J., Jüngert, G., Hiersemenzel, A., Siebler, A., Schmit, F., Öztürk, Y., Dannenmaier, S., et al. (2021a) The universally conserved ATPase YchF regulates translation of leaderless mRNA in response to stress conditions. Front. Mol. Biosci. 8, 643696. DOI: 10.3389/fmolb.2021.643696
- Landwehr, V., Milanov, M., Hong, J., and Koch, H.-G. (2021b) The role of the universally conserved ATPase YchF/Ola1 in translation regulation during cellular stress. Microorganisms 10, 14. DOI: 10.3390/microorganisms10010014
- Lin, Z., Li, R., Han, Z., Liu, Y., Gao, L., Huang, S., Miao, Y., and Miao, R. (2023) The universally conserved unconventional G protein YchF is critical for growth and stress response. Life 13, 1058. DOI: 10.3390/life13041058
- Luo, H., Lin, Y., Liu, T., Lai, F.-L., Zhang, C.-T., Gao, F., and Zhang, R. (2021) DEG 15, an update of the Database of Essential Genes that includes built-in analysis tools. Nucleic Acids Res. 49, D677–D686. DOI: 10.1093/nar/gkaa917
- Luo, M., Han, Z., Huang, G., Li, R., Liu, Y., Lu, J., Liu, L., and Miao, R. (2022) Structural comparison of unconventional G protein YchF with heterotrimeric G protein and small G protein. Plant Signal. Behav. 17, 2024405. DOI: 10.1080/15592324.2021.2024405
- Marlovits, T. C., Haase, W., Herrmann, C., Aller, S. G., and Unger, V. M. (2002) The membrane protein FeoB contains an intramolecular G protein essential for Fe(II) uptake in bacteria. Proc. Natl. Acad. Sci. USA 99, 16243–16248. DOI: 10.1073/pnas.242338299
- Mendler, K., Chen, H., Parks, D. H., Lobb, B., Hug, L. A., and Doxey, A. C. (2019) AnnoTree: visualization and exploration of a functionally annotated microbial tree of life. Nucleic Acids Res. 47, 4442–4448. DOI: 10.1093/nar/gkz246
- Miller, J. H. (1972) Experiments in Molecular Genetics. Cold Spring Harbor Laboratory Press, NY, USA. ISBN: 978-0879691066
- Mistry, J., Bateman, A., and Finn, R. D. (2007) Predicting active site residue annotations in the Pfam database. BMC Bioinformatics 8, 298. DOI: 10.1186/1471-2105-8-298
- Mistry, J., Chuguransky, S., Williams, L., Qureshi, M., Salazar, G. A., Sonnhammer, E. L. L., Tosatto, S. C. E., Paladin, L., Raj, S., Richardson, L. J., et al. (2021) Pfam: the protein families database in 2021. Nucleic Acids Res. 49, D412–D419. DOI: 10.1093/nar/gkaa913
- Mizushima, S., and Nomura, M. (1970) Assembly mapping of 30S ribosomal proteins from E. coli. Nature 226, 1214–1218. DOI: 10.1038/2261214a0
- Park, S., You, X., and Imlay, J. A. (2005) Substantial DNA damage from submicromolar intracellular hydrogen peroxide detected in Hpx- mutants of Escherichia coli. Proc. Natl. Acad. Sci. USA 102, 9317–9322. DOI: 10.1073/pnas.0502051102
- Parks, D. H., Chuvochina, M., Rinke, C., Mussig, A. J., Chaumeil, P.-A., and Hugenholtz, P. (2022) GTDB: an ongoing census of bacterial and archaeal diversity through a phylogenetically consistent, rank normalized and complete genome-based taxonomy. Nucleic Acids Res. 50, D785–D794. DOI: 10.1093/nar/gkab776
- Paysan-Lafosse, T., Blum, M., Chuguransky, S., Grego, T., Pinto, B. L., Salazar, G. A., Bileschi, M. L., Bork, P., Bridge, A., Colwell, L., et al. (2023) InterPro in 2022. Nucleic Acids Res. 51, D418–D427. DOI: 10.1093/nar/gkac993
- Singh, N. S., Ahmad, R., Sangeetha, R., and Varshney, U. (2008) Recycling of ribosomal complexes stalled at the step of elongation in Escherichia coli. J. Mol. Biol. 380, 451–464. DOI: 10.1016/J.JMB.2008.05.033
- Singh, N. S., Das, G., Seshadri, A., Sangeetha, R., and Varshney, U. (2005) Evidence for a role of initiation factor 3 in recycling of ribosomal complexes stalled on mRNAs in Escherichia coli. Nucleic Acids Res. 33, 5591–5601. DOI: 10.1093/nar/gki864
- Singh, R., Wiseman, B., Deemagarn, T., Donald, L. J., Duckworth, H. W., Carpena, X., Fita, I., and Loewen, P. C. (2004) Catalase-peroxidases (KatG) exhibit NADH oxidase activity. J. Biol. Chem. 279, 43098–43106. DOI: 10.1074/jbc.M406374200
- Steinegger, M., and Söding, J. (2017) MMseqs2 enables sensitive protein sequence searching for the analysis of massive data sets. Nat. Biotechnol. 35, 1026–1028. DOI: 10.1038/nbt.3988
- Taoka, M., Morofuji, N., Yamauchi, Y., Ojima, H., Kubota, D., Terukina, G., Nobe, Y., Nakayama, H., Takahashi, N., Kosuge, T., et al. (2014) Global PROTOMAP profiling to search for biomarkers of early-recurrent hepatocellular carcinoma. J. Proteome Res. 13, 4847–4858. DOI: 10.1021/pr500262p
- Taoka, M., Yamauchi, Y., Nobe, Y., Masaki, S., Nakayama, H., Ishikawa, H., Takahashi, N., and Isobe, T. (2009) An analytical platform for mass spectrometry-based identification and chemical analysis of RNA in ribonucleoprotein complexes. Nucleic Acids Res. 37, e140–e140. DOI: 10.1093/nar/gkp732
- Teplyakov, A., Obmolova, G., Chu, S. Y., Toedt, J., Eisenstein, E., Howard, A. J., and Gilliland, G. L. (2003) Crystal structure of the YchF protein reveals binding sites for GTP and nucleic acid. J. Bacteriol. 185, 4031–4037. DOI: 10.1128/JB.185.14.4031-4037.2003
- Tomar, S. K., Kumar, P., and Prakash, B. (2011) Deciphering the catalytic machinery in a universally conserved ribosome binding ATPase YchF. Biochem. Biophys. Res. Commun. 408, 459–464. DOI: 10.1016/j.bbrc.2011.04.052
- Watanabe, K., Tominaga, K., Kitamura, M., and Kato, J.-i. (2016) Systematic identification of synthetic lethal mutations with reduced-genome Escherichia coli: synthetic genetic interactions among yoaA, xthA and holC related to survival from MMS exposure. Genes Genet. Syst. 91, 183–188. DOI: 10.1266/ggs.15-00068
- Wenk, M., Ba, Q., Erichsen, V., MacInnes, K., Wiese, H., Warscheid, B., and Koch, H.-G. (2012) A universally conserved ATPase regulates the oxidative stress response in Escherichia coli. J. Biol. Chem. 287, 43585–43598. DOI: 10.1074/jbc.M112.413070
- Yamazaki, Y., Niki, H., and Kato, J.-i. (2007) Profiling of Escherichia coli chromosome database. Methods Mol. Biol. 416, 385–389. DOI: 10.1007/978-1-59745-321-9_26
- Zhang, J.-w., Rubio, V., Zheng, S., and Shi, Z.-z. (2009) Knockdown of OLA1, a regulator of oxidative stress response, inhibits motility and invasion of breast cancer cells. J. Zhejiang Univ. Sci. B 10, 796–804. DOI: 10.1631/jzus.B0910009








