2014 Volume 89 Issue 1 Pages 27-34
2014 Volume 89 Issue 1 Pages 27-34
The insect steroid hormone ecdysteroid plays pivotal roles in the temporal coordination of development, represented by molting and metamorphosis. During the larval stages, ecdysteroid is biosynthesized from dietary cholesterol by several ecdysteroidogenic enzymes in the specialized endocrine organ called the prothoracic gland (PG). As ecdysteroid biosynthesis in the PG is affected by several environmental cues, such as photoperiod and nutrition, a fundamental question is how the ecdysteroid biosynthesis pathway is controlled in response to environmental cues. In this review, we briefly summarize recent topics on the regulatory mechanisms of ecdysteroid biosynthesis, especially the neuronal regulatory mechanism, in the fruit fly Drosophila melanogaster. The implications from studies with other insects are also discussed.
The development of most model organisms is stereotyped inside a laboratory, although their genetic programs hold enormous potential for adapting to environmental challenges. This property allows animals to increase their survival fitness and reproductive success to accomplish continuity of the species.
The genetic program of development is coordinated in four dimensions, not only along the spatial axes but also along the time-axis. While spatial pattern formation is rigidly programed, the appropriate timing of specific developmental events is changeable in the context of the environment. The latter involves systemic signaling systems that respond to environmental cues, such as nutrient status. One of the key players in this “developmental flexibility” is a steroid hormone (Danielsen et al., 2013; Miller and Auchus, 2011). As steroid hormones are lipid soluble and thus can directly enter most cells through cell membranes from the circulating hemolymph (Miller and Auchus, 2011), they allow a coordinated response throughout the whole body during animal development.
The insect steroid hormone ecdysteroid plays pivotal roles in the temporal coordination of development. The life cycle of insects has several morphologically distinct stages (embryo, larva, pupa, and adult), thus providing a suitable model system for studying developmental transition from one stage to the next (Fig. 1). The developmental transitions, such as molting and metamorphosis, are dictated by pulses of ecdysteroid that in turn activate a battery of gene cascades.
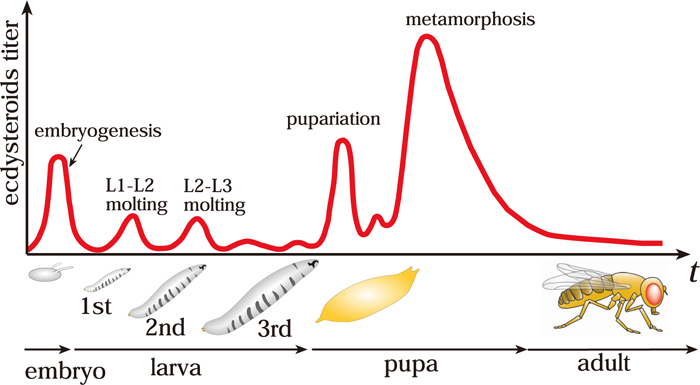
The developmental stages and ecdysteroid titers in D. melanogaster. The X-axis is a timeline of developmental stage, while the Y-axis represents the levels of ecdysteroid titer. Each of the ecdysteroid pulses triggers embryogenesis, molting, pupariation and metamorphosis. The ecdysteroid titer profile is depicted as 20E equivalents in whole body homogenates (Riddiford, 1993).
Because the timing of molting and metamorphosis depends on various environmental cues, such as nutrition, photoperiod, and temperature (Yamanaka et al., 2013a), one of the fundamental questions in insect developmental biology is how ecdysteroid biosynthesis is controlled in response to various environmental cues at the molecular level.
In the last decade, powerful genetic and molecular approaches using the fruit fly Drosophila melanogaster have significantly contributed to the understanding of the molecular basis of the ecdysteroid biosynthesis pathway and its regulatory signaling pathway. While the findings from D. melanogaster shed light on a conserved mechanism in insects, they also reveal the diversity of the hormone regulatory system among the insect species. In this review, we briefly summarize recent topics on the regulatory mechanisms of ecdysteroid biosynthesis, especially the neuronal regulatory mechanism, in the context of various environmental conditions in D. melanogaster.
During the larval stages, ecdysteroids are biosynthesized from dietary cholesterol in the specialized endocrine organ called the prothoracic gland (PG) (Fig. 2; Gilbert et al., 2002; Iga and Kataoka, 2012; Niwa and Niwa, 2011). In the PG, cholesterol is converted into ecdysone (previously called α-ecdysone) through several intermediate steps by a series of ecdysteroidogenic enzymes. Indeed, molecular studies using D. melanogaster as well as the silkworm Bombyx mori have successfully identified several ecdysteroidogenic enzymes, including Neverland (Lang et al., 2012; Yoshiyama et al., 2006; Yoshiyama-Yanagawa et al., 2011), Non-molting glossy/Shroud (Niwa et al., 2010), CYP307A1/Spook (Namiki et al., 2005; Ono et al., 2006), CYP307A2/Spookier (Ono et al., 2006), CYP6T3 (Ou et al., 2011), CYP306A1/Phantom (Niwa et al., 2004; Warren et al., 2004), CYP302A1/Disembodied (Chavez et al., 2000; Niwa et al., 2005; Warren et al., 2002), and CYP315A1/Shadow (Warren et al., 2002). Ecdysone is secreted into the hemolymph and converted into 20-hydroxyecdysone (20E) by another enzyme CYP314A1/Shade (Petryk et al., 2003). 20E is thought to be an active form of ecdysteroid that binds to a nuclear receptor EcR/USP complex. Ecdysteroid signaling initiates various gene expression cascades, which ultimately lead to drastic tissue rearrangements during developmental transitions (Thummel, 2001).
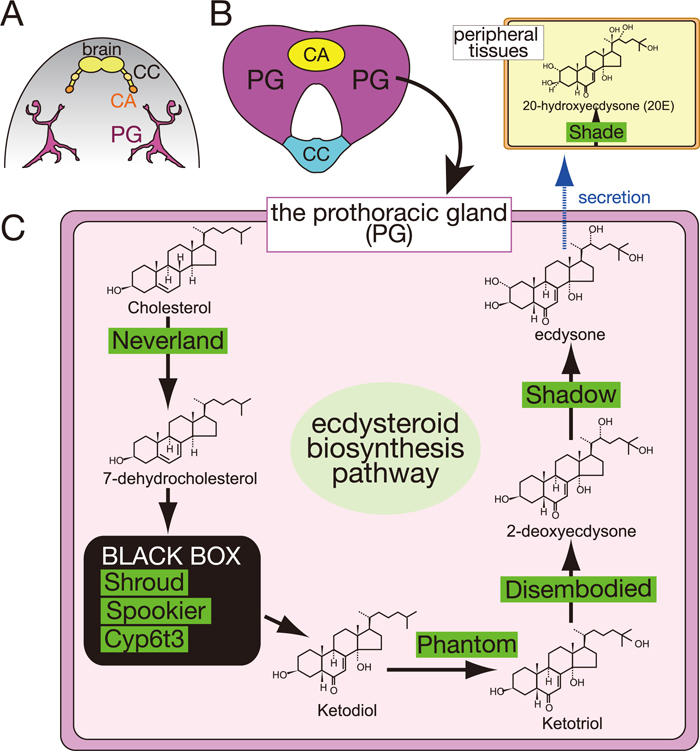
The ecdysteroid biosynthesis pathway in the prothoracic gland. (A and B) Schematic drawings of the endocrine organs: the corpora cardiaca (CC), the corpora allata (CA), and the prothoracic gland (PG) in the larva of the silkworm B. mori (A) and D. melanogaster (B). In flies, the ring gland (RG) contains the PG (magenta), the CA (yellow), and the CC (blue). (C) The ecdysteroid biosynthesis pathway in the PG. Cholesterol is converted into ecdysone (previously called α-ecdysone) by several ecdysteroidogenic enzymes (green box). In the black box, at least three enzymes have been identified, but their intermediates have not been determined. Once ecdysone is secreted into the hemolymph, it is converted into 20E in the peripheral tissues.
In the past, a number of biochemical and physiological studies using large-size insects, such as B. mori and the tobacco hornworm Manduca sexta, succeeded in identifying and characterizing trophic/static factors acting on the PG (Tanaka, 2011). A well-known example is a cerebral neuropeptide called prothoracicotropic hormone (PTTH). PTTH was originally purified from B. mori brain extracts as a substance that could rescue the developmental arrest of artificially diapausing pupae in which their brains are surgically extirpated (Kataoka et al., 1991; Kawakami et al., 1990). In Lepidoptera, two pairs of PTTH-producing neurons project to the glandular cells of the corpora allata (CA) (Fig. 2A; Mizoguchi et al., 1990). Once released from the CA into the hemolymph, PTTH targets the PG and triggers the production of ecdysone via one or more second-messenger pathways, including Ca2+, cAMP, and a MAP kinase cascade (Gilbert et al., 2002; Gu et al., 2010; Rybczynski et al., 2001; Rybczynski and Gilbert, 2003). Because recombinant PTTH is sufficient to induce the expression of ecdysteroidogenic genes in cultured PGs (Namiki et al., 2005; Niwa et al., 2005, 2011; Yamanaka et al., 2007), it has been thought that PTTH is the neurohormone that is the primary regulator of ecdysteroid biosynthesis.
In contrast, in D. melanogaster, PTTH-producing neurons directly innervate the PG but not the CA (Fig. 3A; McBrayer et al., 2007). These neurons have been identified as the “PG-LP” neurons by an enhancer trap screen (Siegmund and Korge, 2001). Surprisingly, despite an expectation from studies using B. mori that PTTH must be essential for insect metamorphosis, genetic ablation of PTTH-producing neurons does not cause mortality in D. melanogaster (McBrayer et al., 2007). Larvae lacking PTTH significantly delay timing of ecdysteroidogenic gene expression and timing of ecdysteroid biosynthesis but eventually pupate. The extended feeding larval period gives rise to giant-size pupae and adults. These phenotypes are also observed in animals with PG-specific RNAi knockdown of the PTTH receptor gene torso (Rewitz et al., 2009). Downstream of Torso, the Ras/MAP kinase pathway is activated, promoting ecdysteroid biosynthesis (Caldwell et al., 2005; Rewitz et al., 2009). These data suggest that D. melanogaster PTTH is required for the precise regulation of ecdysteroidogenesis but is not a crucial time-gatekeeper in the PG. Interestingly, the expression of ptth shows an unusual cyclic pattern with an 8-hour periodicity (McBrayer et al., 2007). As PTTH neurons make synaptic contacts with circadian pacemaker neurons (Gong et al., 2010; Siegmund and Korge, 2001), it is very likely that the expression of ptth is under circadian rhythm control. Further studies will be required to understand the transcriptional regulation of the ptth gene and the light-dependent activity of PTTH neurons. The relationship between developmental timing and the circadian clock remains to be solved.
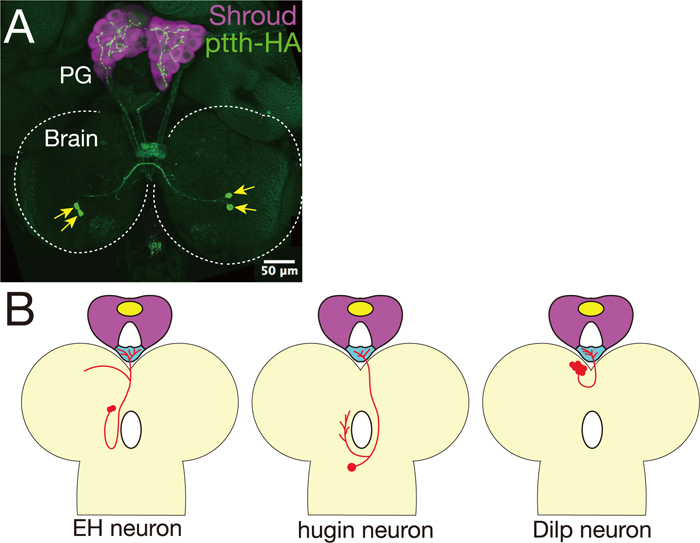
Examples of ring gland-innervating neurons. (A) The brain-RG complex from a 3rd instar larva expressing a genomic fragment of the ptth gene tagged with HA. The PG was labeled with anti-Shroud antibody (Y. S. N. and R. N., unpublished material; magenta) and PTTH-producing neurons were labeled with anti-HA antibody (green). Arrows indicate two pairs of PTTH cells located in the brain. Their axon terminals innervate the PG. (B) Schematic drawings of the CC-innervating neurons in the Br-RG complex: eclosion hormone (EH)-releasing neurons, hugin-expressing neuron, and Drosophila insulin-like peptide (Dilp)-producing neurons.
What are the other signaling pathways that regulate ecdysteroid biosynthesis in the PG? To date, the insulin/insulin-like growth factor signaling (IIS) pathway, TOR pathway, and TGFβ/Activin signaling pathway have been implicated. TGFβ/Activin signaling upregulates the expression of the PTTH receptor gene torso and insulin receptor (InR) gene in the PG (Gibbens et al., 2011). The IIS pathway regulates larval growth rate (Colombani et al., 2005; Mirth et al., 2005), while the TOR pathway affects developmental timing in response to nutrient conditions (Layalle et al., 2008). These multiple signaling pathways may provide a crosscheck mechanism to ensure that both developmental and nutritional inputs are synchronized before initiating the final genetic program leading to adult development.
The timing of ecdysteroid biosynthesis is also affected by growth abnormalities of the imaginal discs, which are primordia of adult structures (Hackney et al., 2012; Parker and Shingleton, 2011; Stieper et al., 2008). When imaginal disc growth is impaired, Dilp8, a Drosophila insulin-like peptide (Dilp) and member of the relaxin family, is secreted from the damaged discs to control developmental timing (Colombani et al., 2012; Garelli et al., 2012). Notably, Dilp8 delays the expression of ecdysteroidogenic genes in the PG, leading to delayed ecdysteroid biosynthesis. This mechanism allows extra time for tissue repair and growth to occur, synchronizing the growth of undamaged tissues with delayed ones. Because the IIS pathway in the PG regulates the growth rate but not developmental timing (Colombani et al., 2005), how Dilp8 inhibits ecdysteroid biosynthesis in the PG remains to be elucidated. In addition to Dilp8, it is predicted that an uncharacterized retinoid-like signaling is also involved in the damage-response regulation of ecdysteroid biosynthesis via PTTH (Halme et al., 2010).
While PTTH neurons are the only neurons in D. melanogaster that have been reported to project to the PG (McBrayer et al., 2007; Siegmund and Korge, 2001), different types of PG-innervating neurons have been reported in other insects, such as the silkworm B. mori (Tanaka, 2011; Yamanaka et al., 2006; Yokoyama, 1956), the cabbage moth Mamestra brassicae (Okajima and Watanabe, 1989) and the American cockroach Periplaneta americana (Richter and Bohm, 1997). The electrophysiological activities of these neurons correlate with the ecdysteroid titer, suggesting that these neurons stimulate the PG with tropic or static factors. In addition, from the study of M. brassicae (Okajima and Watanabe, 1989), both efferent and afferent activities were observed in the PG-innervating neurons, suggesting that a neuronal feedback mechanism is involved in the regulation of ecdysteroid biosynthesis. These observations raise the intriguing possibility that uncharacterized neural regulation of ecdysteroid biosynthesis is also present in D. melanogaster.
In cyclorrhaphous Diptera, including D. melanogaster, the PG is joined together with the CA and the corpora cardiaca (CC) to form a composite endocrine organ, “the ring gland (RG)” (Fig. 2; Dai and Gilbert, 1991). Apart from the PG, the CA and the CC produce juvenile hormones and adipokinetic hormones, respectively (Jones and Jones, 2007; Van der Horst et al., 2001). The genetic ablation of CA or CC cells does not result in fatal growth defects, suggesting that neither CA nor CC cells are crucial for development (Kim and Rulifson, 2004; Lee and Park, 2004; Liu et al., 2009; Riddiford et al., 2010).
The neural innervation of the RG has been reported in the larvae of D. melanogaster (Siegmund and Korge, 2001), the flesh fly Sarcophaga craassipalis (Giebultowicz and Denlinger, 1985), and the blow fly Protophormia terraenovae (Hamanaka et al., 2009). Curiously, at least in D. melanogaster, the number of neurons innervating the CC is more than that of the PG or the CA. The CC-innervating neurons include Dilps-producing neurons (Rulifson et al., 2002), eclosion hormone (EH)-releasing neurons (Horodyski et al., 1993; McNabb et al., 1997), hugin-expressing neurons (Bader et al., 2007; Melcher and Pankratz, 2005), and serotonin-producing neurons (Siegmund and Korge, 2001; Valles and White, 1988; Fig. 3).
In this review, we would like to propose one conjecture: some of the neurons innervating the CC might secrete neuropeptides and/or amines to the PG. This idea is analogous to the silkworm PTTH-producing neurons that project to the CA instead of the PG (Mizoguchi et al., 1990). For example, Dilps-producing neurons innervate the CC, which is located at the most proximal region of the RG to the brain (Rulifson et al., 2002). Dilps might be secreted from the CC, and thereby activate the IIS pathway in the PG. Furthermore, we recently re-examined the projection pattern of the CC-innervating neurites and indeed found that a few processes innervate not only the CC but also the PG (Y. S. N. and R. N., unpublished observation). In the future, the biological significance of these projection patterns should be determined by the functional analysis of individual neurons and by addressing their mechanisms of axon targeting to distinct areas (CC or PG) in the RG.
Another potential key regulator is hugin-expressing neurons that project to not only the CC but also three other distinct targets including the protocerebrum, the ventral nerve cord, and the pharynx (Bader et al., 2007; Melcher and Pankratz, 2005). hugin is expressed in approximately 20 neurons in the subesophageal ganglion (SOG), known as the insect feeding center. The hugin gene encodes a prepropeptide that can give rise to two putative neuropeptides: Pyrokinin-2 (PK-2) and Hug-γ (Melcher and Pankratz, 2005; Meng et al., 2002). Several lines of evidence suggest that these neuropeptides are involved in feeding behavior, molting, and puparium formation (Meng et al., 2002; Verleyen et al., 2004). It is also noteworthy that, besides the hugin-expressing neurons, a subset of serotonergic neurons also innervates not only the RG but also the feeding apparatus such as the pharynx and the proventriculus (Spiess et al., 2008). Because pupariation timing is highly dependent upon nutrient conditions during the larval period, we suppose that these neurons may coordinate feeding/non-feeding behavior with the metabolic status of larval development as well as the larva-to-pupa transition. It would be interesting to further examine whether the CC-innervating neurons affect ecdysteroid biosynthesis in the PG.
As described above, developmental transition is accompanied by many changes in physiological conditions and innate behaviors in response to environmental cues. Therefore, it is feasible that one neuropeptide/neurotransmitter, through a neuroendocrine network, modulates a variety of activities to control ecdysteroid biosynthesis as well as other coordinated events for proper developmental transition. In fact, another function of PTTH as a neurohormone was recently reported in the larva of D. melanogaster. PTTH is released into the hemolymph, acting on the two light sensors (the Bolwig’s organ and the peripheral class IV dendritic arborization neurons) to regulate light avoidance behaviors (Yamanaka et al., 2013b). Therefore, PTTH concomitantly targets different tissues and significantly contributes to the regulation of appropriate development timing and innate behaviors to optimize fitness and survival.
Remarkable advances in techniques and resources have allowed us to explore new directions of research, leading to fruitful findings. The Howard Hughes Medical Institute Janelia GAL4 and the Vienna Drosophila RNAi Center VT line collections will be useful for labeling a small population of neurons innervating the RG or other tissues (Jenett et al., 2012; Pfeiffer et al., 2008). In combination with these strains, bioimaging at high resolution and 3-D reconstruction of neural networks (Sakuma et al., 2014; Hakeda-Suzuki and Suzuki, 2014) will successfully map the precise sites of neural projections. Targeted expression of various transgenes modulating neural activities will classify the functional unit of neural connections. In addition, recently developed novel genome editing technologies (Kondo, 2014) will also be helpful for analyzing gene functions in the PG. The integration of these approaches will reveal the entire neuroendocrine system controlling ecdysteroid biosynthesis and the associated behaviors and physiological changes during the course of developmental transition.
We thank Michael B. O’Connor for providing us with the ptth-HA strain. Y. S. N. is a recipient of fellowships from the Japan Society for the Promotion of Science. This work was supported in part by grants to R. N. from the Inamori Foundation, the Tomizawa Jun-ichi & Keiko Fund of the Molecular Biology Society of Japan for Young Scientists, the Takeda Science Foundation, and the Naito Foundation.