2014 Volume 89 Issue 2 Pages 87-91
2014 Volume 89 Issue 2 Pages 87-91
Peptide signaling plays important roles in various developmental processes of plants. Genes encoding CLE proteins, which are processed into CLE signaling peptides, are required for maintenance of the shoot apical meristem and for vascular differentiation. FON2-LIKE CLE PROTEIN1 (FCP1), a member of the CLE gene family, negatively regulates meristem maintenance in both shoot and root apical meristems of rice (Oryza sativa). Here, we examined the role of FCP1 in leaf development. We found that overexpression of FCP1 affects various aspects of leaf development in shoots regenerated from calli, making it difficult to distinguish between the leaf blade and leaf sheath. Differentiation of tissues such as vascular bundle and sclerenchyma was strongly inhibited by FCP1 overexpression. Spatial expression patterns of developmental genes DROOPING LEAF (DL) and OsPINHEAD1 (OsPNH1) were severely affected in the FCP1-overexpressing shoots. Whereas DL was expressed in the central region of leaf primordia in control shoots, DL expression was expanded throughout the leaf primordia of the FCP1-overexpressing shoots in early developmental stages. By contrast, OsPNH1, which is expressed in provascular and developing vascular tissues in normal seedlings, was strongly repressed by FCP1 overexpression. Taken together, our results suggest that FCP1 is involved in the regulation of cell fate determination during leaf development.
Intercellular communication is particularly important for development in plants, because plant cells are fixed by rigid cell walls and do not migrate. Many types of small peptides are involved in communication between cells and between functional domains within tissues and organs to regulate developmental processes such as stem cell maintenance, vascular differentiation, and stomatal development. In Arabidopsis thaliana, stem cell maintenance in the shoot apical meristem is negatively regulated by a CLAVATA3 (CLV3) CLE peptide, which acts through receptor complexes such as CLV1, CLV2/CRN, and RPK2 (reviewed in Aichinger et al., 2012; Ha et al., 2010; Miyawaki et al., 2013). Mutation in CLV3 leads to an enlarged meristem consisting of over-proliferated stem cells, due to a failure in the repression of WUSCHEL (WUS) activity, which promotes stem cell identity (Fletcher et al., 1999). Conversely, overexpression of CLV3 results in premature termination of the meristem (Brand et al., 2000; Fletcher et al., 1999).
In rice, mutations in the genes FLORAL ORGAN NUMBER1 (FON1) and FON2 give rise to enlargement of the floral meristem, resulting in increases in the numbers of floral organs such as carpels and stamens (Suzaki et al., 2004, 2006). FON1 encodes a CLV1-like LRR receptor kinase, whereas the protein encoded by FON2 contains a CLE domain highly similar to that of Arabidopsis CLV3. Unlike Arabidopsis clv mutants, however, rice fon mutants do not display abnormal phenotypes in the vegetative phase. In addition, overexpression of FON2 does not affect vegetative development, whereas it disturbs inflorescence and flower development probably due to defects in maintenance of the reproductive meristem. Thus, CLV signaling appears to be partially conserved in rice (reviewed in Miyawaki et al., 2013; Pautler et al., 2013).
FON2-LIKE CLE PROTEIN1 (FCP1) encodes a CLE protein closely related to FON2 and plays an important role in the vegetative development of rice (Suzaki et al., 2008; Ohmori et al., 2013). Constitutive overexpression of FCP1 causes premature termination of the meristem in shoots regenerating from calli (Suzaki et al., 2008). Likewise, inducible overexpression of FCP1 results in a failure in meristem maintenance (Ohmori et al., 2013). When FCP1 expression is strongly induced, the undifferentiated cell marker OSH1 and the stem cell marker FON2 are downregulated and leaf initiation is compromised. Conversely, inducible knockdown of FCP1 and FCP2 leads to increased expression of OSH1 and FON2 and expansion of the expression domains of these genes, suggesting that meristem activity is upregulated. Although it is not known whether a WUS ortholog functions similarly in rice, a member of the WUSCHEL-RELATED HOMEO-BOX (WOX) gene family, OsWOX4, is involved in meristem maintenance (Ohmori et al., 2013). Inducible knockdown of OsWOX4 results in downregulation of OSH1 and FON2. Consistent with this observation, the expression of OsWOX4 is negatively regulated by FCP1. Thus, the role of FCP1 in meristem maintenance is relatively well understood. In the current work, we have analyzed the function of FCP1 in other developmental processes such as leaf development and shoot regeneration.
We previously found that overexpression of FCP1 (FCP1-OX) results in the regeneration of abnormal shoots from calli (Suzaki et al., 2008). To examine the role of FCP1 in regeneration, we characterized the regeneration process in detail. Calli were induced from the scutellum and used for Agrobacterium-mediated transformation (Hiei et al., 1994). After selection for hygromycin resistance, calli were transferred to regeneration medium. In calli carrying the empty vector, tiny green spots were observed about 10 d after transfer, and green leaf-like organs were subsequently observed (Fig. 1, A and B). Shoot-like structures and roots were formed after 25 and 30 d on the regeneration medium, respectively (Fig. 1, C and D). Normal shoots, which were very similar in appearance to those of seedlings, were obtained within 40 d (Fig. 1E). By contrast, regeneration was delayed in the FCP1-OX calli. Even after 18 d on the regeneration medium, we observed only green calli that lacked leaf-like structures (Fig. 1, F and G). Many FCP1-OX calli failed to form a shoot-like structure after 30 d (Fig. 1, H and I). After 40 d, shoot-like structures with malformed morphology were apparent, but no root regeneration was observed in the FCP1-OX calli (Fig. 1J). At this stage, we often observed abnormal calli that produced several small, leaf-like organs (Fig. 1K). The shoots that did form on FCP1-OX calli did not grow further and eventually died. Thus, FCP1 overexpression disturbed shoot regeneration from callus and resulted in the production of malformed shoots.
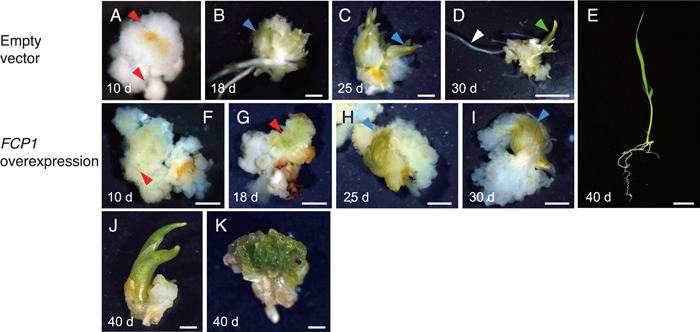
Shoot regeneration from callus. (A–E) Calli carrying empty vector. (F–K) FCP1-OX calli. The number of days after transfer to the regeneration medium is indicated. Arrowheads indicate the following: red, green spot (A, F, G); blue, leaf-like structure (B, C, H, I); green, shoot (D); white, root (D). Scale bars = 1 mm in (A–D, F–K), 1 cm in (E).
To elucidate the effects of FCP1 overexpression, we first made cross-sections of regenerating shoots and observed the inner tissues. Normal leaves were formed in the shoots carrying empty vector, and leaf blades and leaf sheaths were easily distinguished from one another, as in seedlings germinated from seeds (Fig. 2, A and G). The leaf blade contained small cytoplasm-dense cells, whereas the leaf sheath had larger cells that were less stained with toluidine blue. Vascular bundles were differentiated in both the leaf blade and leaf sheath, with large and small vascular bundles distinguishable in the leaf blade (Fig. 2, A and H). In the leaf sheath, sclerenchyma tissues adaxial to the vascular bundles were differentiated and air spaces were formed (Fig. 2H). By contrast, it was hard to distinguish between leaf blade and leaf sheath in the FCP1-OX shoots (Fig. 2D). Although vascular-like structures were sometimes observed in inner leaves, their number and tissue patterns were abnormal. In outer leaves, no vascular-like tissues were formed (Fig. 2, D and I). Sclerenchyma and air spaces were also absent (Fig. 2, D and I). In addition, the morphology of the marginal region was abnormal (Fig. 2D). Taken together, these observations showed that cell differentiation was highly compromised in the FCP1-OX shoots.

Effect of FCP1 overexpression on cell differentiation and expression of marker genes. (A–C, H) Regenerating shoots carrying empty vector. (D–F, I) FCP1-OX regenerating shoots. (G) A shoot germinated from a seed. (A, D) Cross-section of the apex of the regenerating shoots. (B, E) In situ hybridization showing spatial distribution of DL transcripts. (C, F) In situ hybridization showing spatial distribution of OsPNH transcripts. (H, I) Close-up views of the regions indicated with squares in (A) and (D), respectively. (G) Cross-section of the shoot apex of a seedling germinated from a seed. Arrowheads and asterisks indicate vascular bundles and air spaces, respectively. Arrows indicate abnormal margins. Brackets indicate sclerenchyma tissues. LB, leaf blade; LS, leaf sheath; LV, large vascular bundle; SV, small vascular bundle. Scale bars = 100 μm in (A–G), 50 μm in (H, I).
Next, we examined the expression patterns of two genes, DROOPING LEAF (DL) and OsPINHEAD1 (OsPNH1), that are expressed in leaf primordia (Nishimura et al., 2002; Ohmori et al., 2011; Yamaguchi et al., 2004). DL is required for midrib formation in the leaf and is expressed in the central region of the leaf primordium (Ohmori et al., 2011; Yamaguchi et al., 2004). In shoots carrying empty vector, DL transcripts were localized to the central region (Fig. 2B), as in the wild type seedlings. By contrast, DL transcripts were observed in the FCP1-OX shoots throughout the leaf primordia at earlier developmental stages (P2 and P3), but were absent in the leaf primordia at subsequent stages (Fig. 2E). Thus, the spatial and temporal expression pattern of DL was deregulated in the FCP1-OX shoots.
OsPNH1 is responsible for leaf development and meristem maintenance (Nishimura et al., 2002). In the empty-vector control shoots, OsPNH1 was expressed in the presumptive region of vascular differentiation and the adaxial region of the leaf primordia (Fig. 2C), as previously described (Nishimura et al., 2002). By contrast, OsPNH1 expression in the FCP1-OX shoots was not detected in the leaf primordia except for weak expression in a narrow domain of the leaf center (Fig. 2F). These data suggest that overexpression of FCP1 strongly represses OsPNH1 expression in the regenerating shoot.
We previously showed that FCP1 is expressed in the leaf primordia in addition to the shoot apical meristem in wild-type shoot, suggesting that FCP1 functions in leaf development (Suzaki et al., 2008). Our current results from overexpression analysis suggest that FCP1 plays a role in determining cell fate during leaf development. In control regenerating shoots, as in seedlings germinated from seeds, cells differentiate to produce the vascular bundle, sclerenchyma, and marginal tissues. By contrast, cellular differentiation was strongly inhibited in the FCP1-OX shoots. The cells in the FCP1-OX leaf primordia appeared largely homogeneous at a stage when the control had formed leaf sheath cells (Fig. 2, D and I). In addition, air space formation, a process involving programmed cell death in wild-type leaf sheaths (Inada et al., 2002), was absent in the FCP1-OX shoots.
Expression of developmental genes was also altered by FCP1 overexpression, which caused DL and OsPNH1 to be up- and downregulated throughout young leaf primordia, respectively (Fig. 2, E and F). DL promotes cell proliferation in the central region of the leaf primordia in normal seedlings (Ohmori et al., 2011; Yamaguchi et al., 2004). The leaf primordia were thicker in the FCP1-OX shoots than in the control (Fig. 2, A and D), which may result from enhanced cell proliferation caused by ectopic DL expression in the FCP1-OX shoots. In the wild-type shoot, FCP1 is expressed throughout the leaf primordia from the P1 to P3 stage, whereas both DL and OsPNH1 are expressed in specific and distinct regions from P1 to P4 (Nishimura et al., 2002; Ohmori et al., 2011; Yamaguchi et al., 2004). This spatiotemporally overlapping expression pattern between FCP1 and DL/OsPNH1 is consistent with the idea that FCP1 is likely to be involved in the regulation of both DL and OsPNH1 in wild type. We conclude that an appropriate level of FCP1 expression appears to be required for normal leaf development through the function of key developmental factors regulating cell differentiation.
Differentiation of vascular tissues was compromised in FCP1-OX shoots. OsPNH1 was expressed in the presumptive regions of vascular differentiation and developing vascular bundles in the control, whereas this OsPNH1 expression was strongly repressed by FCP1 overexpression (Fig. 2, C and F). It has been reported that antisense suppression of OsPNH1 results in partial failure of vascular differentiation (Nishimura et al., 2002). Therefore, defects in vascular development in the FCP1-overexpressing shoots may be caused by the downregulation of OsPNH1. Alternatively, downregulation of OsWOX4 may be associated with a failure of vascular development in the FCP1-OX shoots. In Arabidopsis, WOX4 plays a crucial role in the maintenance of vascular stem cells (Hirakawa et al., 2010). In rice, we have shown that OsWOX4 is expressed in the provascular and developing vascular tissues, suggesting that OsWOX4 is involved in vascular development as well as meristem maintenance (Ohmori et al., 2013). In addition, OsWOX4 is negatively regulated by FCP1 (Ohmori et al., 2013). It is therefore also plausible that the failure in vascular development in the FCP1-OX shoots is due to downregulation of OsWOX4. These two hypotheses are not mutually exclusive.
Progress in shoot regeneration was delayed in FCP1-OX shoots. This suggests that cell cycle progression or cell proliferation is partially compromised by FCP1 overexpression. This delay may also be related to the disturbance of cell differentiation.
Together, our results suggest that FCP1 is involved in the regulation of cell differentiation in leaf development, likely affecting the expression of many genes. This highlights the importance of understanding FCP1 signaling and its downstream genetic networks in the leaf.
We thank E. Oki for technical assistance. This research was supported in part by Grants-in-Aid for Scientific Research from MEXT (23248001, 25113008 to H.-Y. H.).