2015 Volume 90 Issue 2 Pages 109-114
2015 Volume 90 Issue 2 Pages 109-114
Extracytoplasmic function (ECF) σ factors respond to environmental stresses and regulate numerous genes required for adaptation. Under normal growth conditions, the ECF σ factors are sequestered by transmembrane anti-σ factor proteins, from which they are released under stress conditions. In Bacillus subtilis ugtP null mutant cells, which lack glucolipids, three of the seven ECF σ factors, σM, σV and σX, are activated. The Escherichia coli cell membrane does not contain glucolipids. When the genes for these three ECF σ and anti-σ factors were introduced into E. coli cells, expression of lacZ fused to the ECF σ factor-regulated promoters indicated ECF σ factor activity. Additional expression of the ugtP gene in these E. coli cells led to the synthesis of small amounts of glucolipids, and the activities of σM and σV were repressed, but the activity of σX was unaffected. It is likely that glucolipids directly influence anti-σM and anti-σV factors by stabilizing conformations that sequester the respective ECF σ factors.
The membrane lipid composition of Bacillus subtilis is complex. It includes acidic phospholipids (phosphatidylglycerol [PG] and cardiolipin [CL]), zwitterionic phospholipids (phosphatidylethanolamine [PE] and lysylphosphatidylglycerol [LysPG]) and glucolipids (monoglucosyldiacylglycerol [MGDG], diglucosyldiacylglycerol [DGDG] and triglucosyldiacylglycerol [TGDG]) (de Mendoza et al., 2002; Matsumoto et al., 2012). In contrast, the membrane composition of Escherichia coli is much simpler: it comprises only the three major phospholipids, PG, CL and PE, except for the outer leaflet of the outer membrane which is composed of lipopolysaccharide (Shibuya, 1992). B. subtilis glucolipids are synthesized by the gene product of ugtP, which processively transfers glucose from UDP-glucose to diacylglycerol (Jorasch et al., 1998). MGDG, DGDG and TGDG amount to 1.2%, 9.8% and 0.3%, respectively, of total membrane lipids (Kawai et al., 2006). A ugtP null mutant lacking membrane glucolipids shows abnormal cell morphology (Price et al., 1997; Matsuoka et al., 2011a) and constitutive activation of three extracytoplasmic function (ECF) σ factors, σM, σV and σX (Salzberg and Helmann, 2008; Matsuoka et al., 2011a; Hashimoto et al., 2013).
ECF σ factors respond to environmental stresses and direct the transcription of genes involved in tasks such as the maintenance of cell envelope integrity. B. subtilis has seven ECF σ factors: σM, σV, σW, σX, σY, σZ and σYlaC. Except for σZ, these are regulated directly by their respective cognate transmembrane anti-σ factors, which sequester the σ factors. The genes for the σ factors and the anti-σ factors form operons whose transcription is directed by the cognate σ factors; the activity of the promoter of an ECF σ factor gene is thus indicative of the activity of the respective ECF σ factor (Helmann, 2002; Yoshimura et al., 2004; Asai et al., 2005).
In this study, we introduced σ factor–anti-σ factor operons for σM, σV and σX into E. coli cells and monitored the σ factor activity via lacZ fusion to the promoters of the operons. Glucolipids were synthesized in this heterologous system, and their influence on the activity of the ECF σ factors was analyzed. A heterologous system offers the advantage of allowing the introduced components to be analyzed independently of other effects that might disturb their interactions in the native system.
The bacterial strains and plasmids used in this study are listed in Table 1. Luria-Bertani (LB) medium (Miller, 1992) was used with addition of antibiotics, where appropriate, at the following concentrations (μg ml–1): ampicillin, 50; chloramphenicol, 5; kanamycin, 5; tetracycline, 10; spectinomycin, 100. Cell growth was monitored with a mini photo 518R photometer (TAITEC) equipped with a 530-nm interference filter and a 14-mm light path adaptor or a Klett-Summerson colorimeter equipped with a no. 54 filter.
| Strain or plasmid | Relevant genotype or description | Construction, source or referencea |
|---|---|---|
| E. coli | ||
| DH5α | recA1 gyrA relA1 thi-1 glnV44 hsdR17 endA1 Δ(lacIZYA-argF)U169deoR (ϕ80dlac Δ(lacZ)M15) | Laboratory collection |
| B. subtilis | ||
| 168 | trpC2 | Laboratory collection |
| YTB019 | 168 ugtP::kan | (Matsuoka et al., 2011b) |
| MBS001 | YTB019 aprE::(Pspac-ugtP spc) | pAPNC-ugtP → YTB019 |
| SK71 | trpC2 lys-1 aprEΔ3 nprE18 nprR2 amyE::(PsigM′-lacZ cat) | (Kosono et al., 2004) |
| SK72 | trpC2 lys-1 aprEΔ3 nprE18 nprR2 amyE::(PsigV-lacZ cat) | (Kosono et al., 2004) |
| SK74 | trpC2 lys-1 aprEΔ3 nprE18 nprR2 amyE::(PsigX′-lacZ cat) | (Kosono et al., 2004) |
| YTB004 | 168 amyE::(PsigM′-lacZ cat) | SK71 → 168 |
| YTB041 | 168 amyE::(PsigV-lacZ cat) | SK72 → 168 |
| YTB033 | 168 amyE::(PsigX′-lacZ cat) | SK74 → 168 |
| YTB002 | YTB019 amyE::(PsigM′-lacZ cat) | SK71 → YTB019 |
| YTB040 | YTB019 amyE::(PsigV-lacZ cat) | SK72 → YTB019 |
| YTB030 | YTB019 amyE::(PsigX′-lacZ cat) | SK74 → YTB019 |
| YTB020 | MBS001 amyE::(PsigM′-lacZ cat) | SK71 → MBS001 |
| RMB001 | MBS001 amyE::(PsigV-lacZ cat) | SK72 → MBS001 |
| YTB027 | MBS001 amyE::(PsigX′-lacZ cat) | SK74 → MBS001 |
| Plasmids | ||
| pAPNC213 | aprE ′specr lacI Pspac ′aprE ampr | (Morimoto et al., 2002) |
| pAPNC-ugtP | pAPNC213 Pspac-ugtP | This study |
| pFZY1 | F′lac replicon, lacZY ampr | (Koop et al., 1987) |
| pFZY1-PsigM′ | pFZY1 PsigM′-lacZY | This study |
| pFZY1-PsigV | pFZY1 PsigV-lacZY | This study |
| pFZY1-PsigX′ | pFZY1 PsigX′-lacZY | This study |
| pBR322 | ampr tetr | (Balbás et al., 1986) |
| pBR-sigM-antisigM | pBR322 PsigM′-sigM-yhdL-yhdK tetr | This study |
| pBR-sigV-antisigV | pBR322 PsigV-sigV-rsiV tetr | This study |
| pBR-sigX-antisigX | pBR322 PsigX′-sigX-rsiX tetr | This study |
| pHR718 | pSC101 replicon, lacIq Ptrc specr | (Shiba et al., 2004) |
| pHR718-ugtP-his | pHR718 Ptrc-ugtP-his6 | This study |
First we confirmed the activation of σM, σV and σX in the B. subtilis ugtP null mutant. Activities of σM, σV and σX factors in B. subtilis were monitored by measuring β-galactosidase activities expressed from transcriptional lacZ fusions to the promoters of the operons of the genes for the ECF σ factors and the anti-σ factors, which are autoregulated by the respective σ factors. The lacZ fusions were integrated into the chromosomal amyE region. The β-galactosidase assay method using o-nitrophenyl-β-d-galactoside as substrate and the unit definition were as described by Wang and Doi (1984). As we previously reported (Hashimoto et al., 2013), σM, σV and σX factors were activated in the ugtP mutant cells: 6.9-fold, 3.4-fold and 1.5-fold, respectively (Fig. 1). When the ugtP gene was placed under the control of the isopropyl-thio-β-d-galactoside (IPTG)-inducible promoter Pspac and integrated into the aprE region of the ugtP null mutant, IPTG induction of this ectopic ugtP gene decreased the activities of the ECF σ factors (Fig. 1). Thus the activation of σM, σV and σX in the ugtP null mutant was complemented by an ectopic copy of ugtP, indicating that it is the defect of ugtP itself, not a polar effect of the ugtP disruption, that is responsible for the activation of the σ factors.

Activation of σM, σV and σX in the B. subtilis ugtP null mutant and its suppression by ectopic expression of ugtP. The strains used were YTB004 (WT), YTB002 (ugtP) and YTB020 (Pspac-ugtP) in A, YTB041 (WT), YTB040 (ugtP) and RMB001 (Pspac-ugtP) in B, and YTB033 (WT), YTB030 (ugtP) and YTB027 (Pspac-ugtP) in C. Overnight cultures were diluted 100-fold in LB medium containing either no IPTG or the indicated concentration of IPTG. After 2 h cultivation with shaking, β-galactosidase activity was measured. The means and standard errors of three measurements are shown.
To monitor the activities of σM, σV and σX in E. coli, the promoters of the operons of genes for the ECF σ factors and their anti-σ factors were cloned into a promoter-probe mini-F vector, pFZY1 (Koop et al., 1987), to construct transcriptional lacZ fusions. For construction of plasmids, see Supplementary Information. The plasmids were introduced into the lacZ-defective E. coli strain DH5α. Empty vectors, pBR322 (Balbás et al., 1986) and pHR718 (Shiba et al., 2004), for the plasmids used in the subsequent experiments were also introduced. In these transformants a small amount of β-galactosidase activity (background) was detected (Fig. 2). This activity may be due to transcription by σ70-containing RNA polymerase of E. coli.
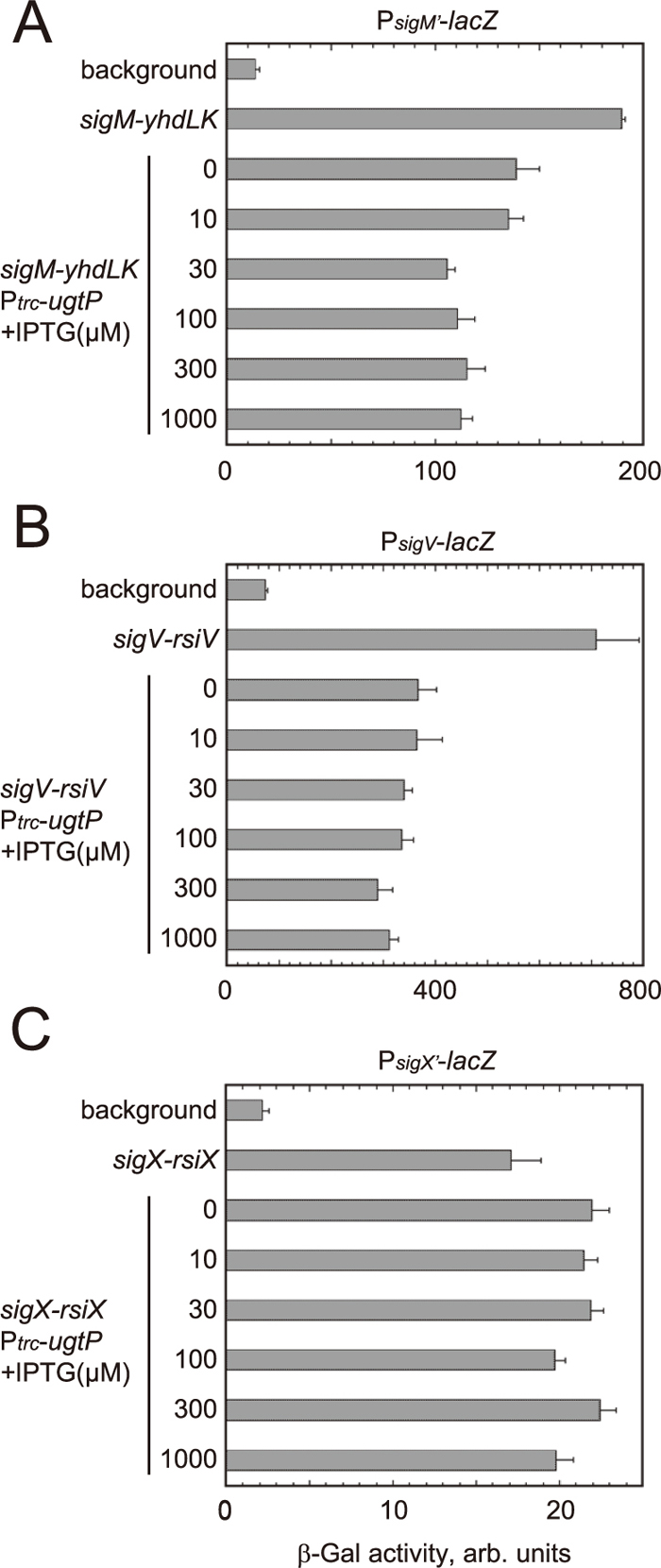
Activity of σM, σV and σX in E. coli cells and the effect of expression of ugtP. The strain used for background in each panel was DH5α harboring pFZY1-PsigM′ and empty vectors pBR322 and pHR718 (A), pFZY1-PsigV, pBR322 and pHR718 (B), or pFZY1-PsigX′, pBR322 and pHR718 (C). The strain used for the second bar of each panel was DH5α harboring pFZY1-PsigM′, pBR-sigM-antisigM and the empty vector pHR718 (A), pFZY1- PsigV, pBR-sigV-antisigV and pHR718 (B), or pFZY1-PsigX′, pBR-sigX-antisigX and pHR718 (C). The strain used with increasing concentrations of IPTG was DH5α harboring pFZY1-PsigM′, pBR-sigM-antisigM and pHR718-ugtP-his (A), pFZY1-PsigV, pBR-sigV-antisigV and pHR718-ugtP-his (B), or pFZY1-PsigX′, pBR-sigX-antisigX and pHR718-ugtP-his (C). Overnight cultures were diluted 100-fold in LB medium containing either no IPTG or the indicated concentration of IPTG and cultivated with shaking. At 0.25 on the mini photo 518R photometer, β-galactosidase activity was measured. The means and standard errors of three measurements are shown.
Next, pBR322-derived multicopy plasmids carrying the ECF σ factor–anti-σ factor operons and the empty vector pHR718 were introduced. Introduction of a plasmid carrying the genes for σM and anti-σM factors (YhdL and YhdK; σM has two anti-σ factors) led to a more than 10-fold increase in β-galactosidase activity compared with the background activity (Fig. 2A). This increment corresponds to the σM activity in the absence of glucolipid since the E. coli membrane does not contain glucolipid. E. coli RNA polymerase core enzyme seems to form holoenzyme with B. subtilis σM. Further introduction of the ugtP gene under the control of the IPTG-inducible promoter Ptrc on pHR718 reduced the activity to ca. 75% even in the absence of IPTG. Addition of IPTG at concentrations of >30 μM reduced the activity to ca. 60% of that in the absence of the ugtP gene. Reduction in the activity in the presence of pHR718-ugtP-his can most likely be ascribed to anti-σM factors that sequester σM in the presence of glucolipids.
Introduction of a plasmid carrying the genes for σV and anti-σV factor (RsiV) led to a 10-fold increase in β-galactosidase activity compared with the background activity (Fig. 2B). E. coli RNA polymerase core enzyme seems to form holoenzyme with B. subtilis σV. Further introduction of the plasmid carrying the ugtP gene under the control of Ptrc reduced the activity to ca. 50% even in the absence of IPTG. Addition of IPTG slightly reduced the activity. These results indicate that anti-σV factor sequesters σV in the presence of glucolipids and that the function is weakened in the absence of glucolipids.
Introduction of a plasmid carrying the genes for σX and anti-σX factor (RsiX) led to an eight-fold increase in β-galactosidase activity compared with the background activity (Fig. 2C). E. coli RNA polymerase core enzyme seems to form holoenzyme with B. subtilis σX. However, additional introduction of a plasmid carrying the ugtP gene did not reduce the activity even after addition of IPTG; instead, it slightly increased the activity. The presence of glucolipids did not strengthen the function of anti-σX factor in E. coli.
Repression of the activity of σM and σV by ugtP expression in E. coli was also confirmed by real-time RT-PCR (see Supplementary Information and Supplementary Fig. S1).
We tried to clone the genes for the ECF σ factors without the genes for the cognate anti-σ factors. For cloning procedures, see Supplementary Information. Repeated attempts to do so, however, were unsuccessful. The anti-σ factors are not likely to completely lose their ability to sequester the cognate σ factors even in the absence of glucolipids. In the absence of anti-σ factors large amounts of the ECF σ factors, whose genes are positively autoregulated on multicopy plasmids, would freely interact with RNA polymerase core enzyme and cause a shortage of σ70-containing holoenzyme. We suppose that this was the reason why we could not clone the genes for the ECF σ factors alone.
Lipid compositions of the strains used for the assay of the ECF σ activities were analyzed by continuous labeling with [14C]acetic acid followed by two-dimensional thin layer chromatography of the extracted lipids as described (Hashimoto et al., 2013) (Fig. 3, Table 2). In these experiments, the ugtP gene was expressed in E. coli under the control of an IPTG-inducible Ptrc promoter using pHR718-ugtP-his, as in Fig. 2. At IPTG concentrations above 100 μM, glucolipid content ceased to increase, and even at 1 mM it was only about 1% in total. This is much lower than that in wild-type B. subtilis cells (ca. 10%). The ratio of MGDG/DGDG/TGDG is also markedly different from that in wild-type B. subtilis cells (1.2%/9.8%/0.3%) (Kawai et al., 2006). MGDG content was below a detectable level even in the presence of 1 mM IPTG, which is consistent with the report by Jorasch et al. (1998), who expressed ugtP (formerly called ypfP) in E. coli.

Two-dimensional thin layer chromatography of lipids of E. coli cells expressing ugtP. Cells were labeled with 3.75 × 104 Bq ml–1 of [1-14C]acetic acid (Amersham; 2.12 × 109 Bq mmol–1) in LB medium at 37 ℃, continuously from initial inoculation for preculture to cell harvesting. At about 100 Klett units, cells were harvested, and lipids were extracted and analyzed by two-dimensional thin layer chromatography as described (Hashimoto et al., 2013). Shown here as examples are the results of DH5α harboring pFZY1-PsigM′, pBR-sigM-antisigM and pHR718 (A), and pFZY1-PsigM′, pBR-sigM-antisigM and pHR718-ugtP-his grown in the presence of 1 mM IPTG (B). CDP-DG is CDP-diacylglycerol.
| DH5α harboring: | IPTG (μM) | mol%a | ||||||
|---|---|---|---|---|---|---|---|---|
| PE | PG | CL | CDP-DG | MGDG | DGDG | TGDG | ||
| pFZY1-PsigM′, pBR-sigM-antisigM and pHR718 | 0 | 80.21 | 15.99 | 3.58 | 0.23 | <0.05 | <0.05 | <0.05 |
| pFZY1-PsigM′, pBR-sigM-antisigM and pHR718-ugtP-his | 0 | 80.81 | 15.65 | 3.30 | 0.24 | <0.05 | <0.05 | <0.05 |
| 10 | 81.28 | 15.71 | 2.77 | 0.19 | <0.05 | 0.05 | <0.05 | |
| 30 | 79.87 | 16.43 | 3.25 | 0.17 | <0.05 | 0.22 | 0.06 | |
| 100 | 79.77 | 15.80 | 3.49 | 0.13 | <0.05 | 0.52 | 0.30 | |
| 300 | 82.23 | 13.71 | 2.87 | 0.15 | <0.05 | 0.59 | 0.45 | |
| 1000 | 82.57 | 12.99 | 2.97 | 0.26 | <0.05 | 0.62 | 0.59 | |
| pFZY1-PsigV, pBR-sigV-antisigV and pHR718 | 0 | 79.95 | 16.39 | 3.44 | 0.22 | <0.05 | <0.05 | <0.05 |
| pFZY1-PsigV, pBR-sigV-antisigV and pHR718-ugtP-his | 0 | 80.75 | 16.09 | 2.89 | 0.26 | <0.05 | <0.05 | <0.05 |
| 10 | 81.56 | 15.50 | 2.65 | 0.25 | <0.05 | <0.05 | <0.05 | |
| 30 | 80.77 | 15.74 | 2.99 | 0.22 | <0.05 | 0.05 | <0.05 | |
| 100 | 81.43 | 14.62 | 2.72 | 0.21 | <0.05 | 0.58 | 0.44 | |
| 300 | 81.49 | 14.18 | 3.11 | 0.22 | <0.05 | 0.58 | 0.42 | |
| 1000 | 81.41 | 14.47 | 2.88 | 0.20 | <0.05 | 0.58 | 0.46 | |
| pFZY1-PsigX′, pBR-sigX-antisigX and pHR718 | 0 | 79.39 | 16.21 | 4.15 | 0.25 | <0.05 | <0.05 | <0.05 |
| pFZY1-PsigX′, pBR-sigX-antisigX and pHR718-ugtP-his | 0 | 80.53 | 14.82 | 4.32 | 0.33 | <0.05 | <0.05 | <0.05 |
| 10 | 80.05 | 15.62 | 4.02 | 0.25 | <0.05 | 0.05 | <0.05 | |
| 30 | 82.27 | 13.19 | 4.09 | 0.29 | <0.05 | 0.11 | 0.05 | |
| 100 | 81.54 | 13.73 | 3.72 | 0.28 | <0.05 | 0.40 | 0.33 | |
| 300 | 82.83 | 12.22 | 3.99 | 0.28 | <0.05 | 0.32 | 0.37 | |
| 1000 | 82.14 | 13.04 | 3.75 | 0.26 | <0.05 | 0.37 | 0.44 | |
If the glucolipid content could be further increased in E. coli, a higher level of repression of σM and σV activities might be observed. However, as shown in Fig. 2, a considerable level of repression was observed even without addition of IPTG in E. coli. In the absence of IPTG there would be leaky expression from the Ptrc promoter, but the glucolipid content was below a detectable level. With increasing concentrations of IPTG to 100 μM, glucolipid content also increased, and the repression levels of σM and σV activities were slightly higher. We surmise that a low glucolipid content suffices to potentiate anti-σM and anti-σV factors to a considerable level.
In E. coli cells, heterologous σM and σV from B. subtilis showed higher activity in the absence of glucolipids than in their presence. This parallels the activation of these σ factors in the ugtP mutant of B. subtilis (Matsuoka et al., 2011a; Hashimoto et al., 2013). Glucolipids are most likely incorporated into the membrane in E. coli cells, and the anti-σ factors are canonical membrane proteins with hydrophobic transmembrane segments and presumably behave as membrane proteins in E. coli cells. We suggest that transmembrane anti-σ factors are potentiated to sequester the σ factors by the presence of glucolipids. Since glucolipids in the heterologous E. coli cells exhibited the same effect as in the B. subtilis cells, we infer that glucolipids probably directly influence anti-σM and anti-σV factors. Glucolipids probably help the anti-σ factors to maintain conformations suitable for sequestering the respective ECF σ factors. In the absence of glucolipids, anti-σ factors cannot stabilize the conformations, and the ECF σ factors are released and allowed to associate with RNA polymerase core enzyme. In this sense, glucolipids may have a chaperone-like function. It has been reported that PE acts as a chaperone in the assembly of the lactose permease LacY in E. coli (Bogdanov et al., 1996).
In contrast, the activity of σX was not repressed by the presence of glucolipids in E. coli. In fact, its activity was slightly increased in their presence; the reason for this is not known. In B. subtilis cells some additional factor(s), which are absent in E. coli, may be involved in the regulation of anti-σX factor function by glucolipids.
It has been reported that the activation of σV of B. subtilis involves regulated intramembrane proteolysis (RIP) of the anti-σV factor (Hastie et al., 2013). When B. subtilis cells are challenged by lysozyme, lysozyme-bound anti-σV factor is cleaved in its extracytoplasmic domain by a signal peptidase (Hastie et al., 2014), and intramembrane proteolysis by the site 2 protease RasP follows (Hastie et al., 2013). The activation of σV in the absence of glucolipids in E. coli may involve RIP by E. coli proteases. Alternatively, a conformational change of anti-σV may activate σV weakly: σV activation in the absence of glucolipids is only several-fold, whereas lysozyme treatment causes 65-fold activation (Hastie et al., 2013).
This study was supported in part by a Grant-in-Aid for Scientific Research (C) from the Japan Society for the Promotion of Science.