2015 Volume 90 Issue 2 Pages 115-120
2015 Volume 90 Issue 2 Pages 115-120
Introgression between related species with different ploidy levels has played important roles in wheat subspecies differentiation. Persian wheat, a cultivated tetraploid wheat subspecies (Triticum turgidum subsp. carthlicum), is postulated to have evolved through interploidy hybridization between tetraploid and hexaploid wheats. Here, we report evidence for the origin of subsp. carthlicum based on the discovery of a new allele for the 5th-to-6th exon region of the Wknox1bKNOTTED1-type homeobox gene in a common wheat subspecies (T. aestivum subsp. carthlicoides). In this Wknox1b region, subsp. carthlicoides contains an inverted duplication mutation in the 3’ flanking region of a 157-bp MITE insertion site. This structural mutation resulted in the suppression of Wknox1b expression in subsp. carthlicoides, but no structural mutation was observed in the same region of subsp. carthlicum. In addition, the carthlicum allele for the Wknox1b 5th-to-6th exon region exhibited the same sequence as that in the wild emmer wheat subsp. dicoccoides. These observations support an alternative hypothesis that subsp. carthlicum evolved by interploidy hybridization between subsp. carthlicoides and tetraploid wheat.
Polyploid wheat species evolved through natural hybridization and allopolyploidization, and the subsequent interploidy introgression between tetraploid and hexaploid wheat species was at least partly associated with subspecies diversification (Matsuoka, 2011). In cultivated tetraploid wheat, interploidy introgression is assumed to have contributed to the birth of two subspecies, Persian wheat, Triticum turgidum subsp. carthlicum (Neyski) Á. Löve & D. Löve (syn. T. persicum Vav.), and Georgian wheat, T. turgidum subsp. paleocholchicum (Menabde) Á. Löve & D. Löve. Subspecies carthlicum belongs to the cultivated tetraploid wheat group having the A and B genomes, and its cultivation area is distributed in the Transcaucasus region in countries such as Georgia and Armenia (van Slageren, 1994). Subspecies carthlicum is morphologically characterized by a long awn at an empty glume, controlled by the tetraaristatus (four-awned) gene on the long arm of chromosome 5A (Haque et al., 2011). The spike morphology of subsp. carthlicum resembles that of common wheat, T. aestivum L., rather than that of other subspecies of free-threshing tetraploid wheat. Moreover, the morphology of synthetic hexaploid wheat derived from crosses between subsp. carthlicum and Aegilops tauschii Coss., the D genome progenitor of common wheat, resembles that of common wheat (Kihara et al., 1950). Therefore, subsp. carthlicum is a candidate for the AB-genome donor of common wheat, whereas it is also considered to be a secondary species derived from an interspecific cross between emmer and common wheat (Vavilov, 1926, cited by Ohtsuka (1991)). Kuckuck (1979) found hexaploid wheat accessions showing the subsp. carthlicum-like morphology, and these accessions, called T. aestivum subsp. carthlicoides nom. nud., were distributed in the border region of Iran, Turkey and the Transcaucasus. Subspecies carthlicum was proposed to have originated from spontaneous hybridization between subsp. carthlicoides and cultivated emmer wheat, T. turgidum subsp. dicoccon (Schrank) Thell. (Kuckuck, 1979).
A wheat ortholog of maize knotted1 (kn1) and rice OSH1, Wknox1, belongs to the class I KN1-type homeobox (KNOX) gene family on the homoeologous group 4 chromosomes (Takumi et al., 2000), which function in maintenance of shoot apical meristem (SAM) activity, determination of cell fate and vegetative organ development (Lincoln et al., 1994; Smith et al., 1995; Kerstetter et al., 1997). Our previous study showed that the three Wknox1 homoeologous loci are functionally conserved in the three component genomes of common wheat, although many structural mutations containing MITE insertions in intron sequences have accumulated in each homoeoallele during wheat polyploid evolution (Morimoto et al., 2005). Here, we report structural mutations newly found at the B-genome locus of Wknox1, designated Wknox1b, in subsp. carthlicoides. Based on a comparison of the carthlicoides Wknox1b allele with other alleles in related species, we discuss the evolution of subsp. carthlicum.
Cytological and molecular distinction of the two subspecies carthlicum and carthlicoidesTen tetraploid accessions of T. turgidum (AABB) subsp. carthlicum, including KU-138, KU-139-1, KU-139-2, KU-187, KU-1800, KU-1801, KU-1807, KU-1808, CGN04221 and CGN08357, and two hexaploid accessions of T. aestivum L. (AABBDD) subsp. carthlicoides, KU-3724 and CGN08360, were used in this study. Plants were grown in a glasshouse of Kobe University for verification of species classification. Somatic chromosome numbers were determined from root-tip mitotic preparations of three seedlings from each accession of subsp. carthlicum and subsp. carthlicoides using the standard acetocarmine squash method. The morphology of the two carthlicoides accessions resembled that of subsp. carthlicum, as previously reported (Kuckuck, 1979; Haque et al., 2011), and the carthlicoides accessions had long awns at empty glumes, especially in late-emerging spikes. To validate ploidy levels of the subsp. carthlicum and subsp. carthlicoides accessions, we first checked chromosome numbers in root tips. Somatic chromosome numbers were 28 in all examined seeds from each accession of subsp. carthlicum, whereas the two carthlicoides accessions had 42 chromosomes.
The genomic sequences of the Wknox1d 4th intron regions were amplified by PCR using the primer pair 5’-AAAAAAAAGGTTAAATGGAC-3’ and 5’-ACCTTATACATGATTGGGAA-3’. An insertion and deletion (indel) mutation site of the Wknox1d locus was previously described (Morimoto et al., 2005), and the primer positions were set in the regions flanking this site. This primer pair precisely recognized and amplified the target region from the ancestral species of common wheat. A common wheat, T. aestivum cv. Chinese Spring (CS), and a durum wheat, T. turgidum subsp. durum cv. Langdon (Ldn), were also used for PCR. PCR was performed according to our previous study (Morimoto et al., 2005), and the amplified DNA fragments were visualized by ethidium bromide staining after electrophoresis on 1.5% agarose gels. In the 4th intron of Wknox1d in common wheat, a 122-bp MITE insertion has been reported (Morimoto et al., 2005). The MITE-containing band was missing in tetraploid wheat accessions including all those examined of subsp. carthlicum (Fig. 1). The Wknox1d-specific MITE insertion was also observed in the two carthlicoides accessions, indicating the presence of the D genome in subsp. carthlicoides as well as in other subspecies of common wheat.
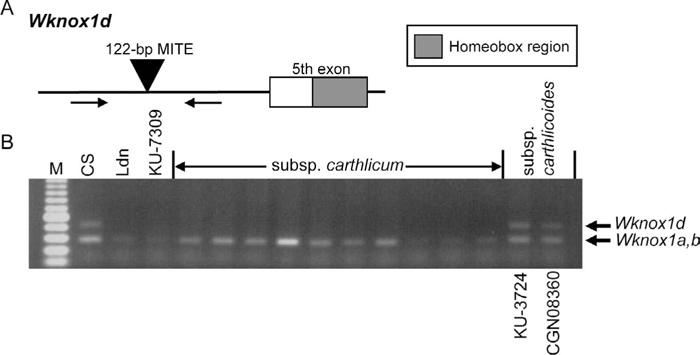
PCR-based haplotype analysis of the 4th intron of Wknox1d. (A) Wknox1d-specific primer positions in the 4th intron and 5th exon region. Arrows indicate the position of PCR primers. A 122-bp MITE insertion specifically observed at the D-genome locus of Wknox1 generates the upper bands in hexaploid wheat accessions. (B) PCR amplification in 10 carthlicum accessions and two carthlicoides accessions. Arrows indicate PCR-amplified fragments. KU-7309 is an accession of T. turgidum subsp. dicoccon. Ldn, T. turgidum subsp. durum cv. Langdon; CS, T. aestivum cv. Chinese Spring.
Similarly to the amplified region of Wknox1d, the Wknox1b 5th-to-6th exon region was amplified by PCR using the primer pair 5’-GCTGAAGCACCATCTCCTGA-3’ and 5’-CATGTAGAAGGCGGCGTTAG-3’. In addition to CS and Ldn, 10 accessions of wild tetraploid wheat, T. turgidum subsp. dicoccoides, and nine accessions of cultivated tetraploid wheat, T. turgidum subsp. dicoccon, were also used to analyze the 157-bp MITE indel polymorphism reported at the 5th intron of Wknox1b in tetraploid wheat (Fig. 2A; Morimoto et al., 2005). The accession numbers of the wild and cultivated tetraploid wheats are in our previous report (Morimoto et al., 2005). Common wheat and cultivated tetraploid wheat cultivars including the subsp. dicoccon accessions contained the MITE insertion in the Wknox1b 5th-to-6th exon region, whereas no fragments containing this insertion were observed in the subsp. dicoccoides accessions (Fig. 2B). The A- and D-genome progenitors T. urartu and Ae. tauschii, respectively, had missing Wknox1b-derived bands, and the subsp. carthlicum accessions showed the same-sized band, without the MITE insertion in the Wknox1b locus, as Ae. speltoides (Fig. 2C). The amplified fragments in the two carthlicoides accessions were clearly longer than those in either subsp. carthlicum or Ldn (Fig. 2, C and D). These observations indicated that the carthlicoides accessions contain a novel allele in the Wknox1b 5th-to-6th exon region.

PCR-based haplotype analysis of the 5th-to-6th exon region of Wknox1b. A 157-bp MITE insertion generates the larger bands in T. turgidum subsp. dicoccon, Ldn and hexaploid wheat accessions. (A) Wknox1b-specific primer positions in the 5th-to-6th exon region. Arrows indicate the position of PCR primers. (B) PCR amplification in wild and cultivated emmer wheat accessions. Arrows indicate PCR-amplified fragments. (C) PCR amplification in the diploid progenitors, 10 carthlicum accessions and two carthlicoides accessions. Arrows indicate PCR-amplified fragments. (D) Comparison of three types of PCR-amplified fragments. Ldn, T. turgidum subsp. durum cv. Langdon; CS, T. aestivum cv. Chinese Spring; ura, T. urartu KU-199-1; spl, Ae. speltoides KU-1-2; tau, Ae. tauschii KU-20-1.
The PCR fragments of the genomic regions near the Wknox1b 5th-to-6th exon in subsp. carthlicum and subsp. carthlicoides were cloned into the vector pGEM-T (Promega, Madison, WI, USA), and the nucleotide sequences were determined by an automated fluorescent DyeDeoxy terminator cycle sequencing system using an ABI PRISM 310 genetic analyzer (Applied Biosystems, Foster City, CA, USA). The nucleotide sequences of the inverted repeat region (see below) were determined using a CUGA sequencing system (Nippon Gene, Tokyo, Japan). The DNA sequences were analyzed by DNASIS software (Hitachi, Tokyo, Japan) and compared with those in other tetraploid and hexaploid wheats. The carthlicoides accessions contained the 157-bp MITE insertion in the 5th intron of Wknox1b, and the inserted MITE sequences were identical to those in CS, Ldn and the subsp. dicoccon accessions (Fig. 3). The genomic region from the 3’ end of the MITE sequence to the 6th exon was inverted in the carthlicoides accessions, and the inverted region contained a 1-bp insertion and a 13-bp deletion at the 5’ end. This inversion significantly disrupted the structure of the homeobox region encoding the DNA-binding motif, and made it impossible to form a precise homeodomain. Additionally, the central region of the 6th exon was duplicated in the carthlicoides accessions, and this duplication resulted in the appearance of longer PCR-amplified fragments for the Wknox1b 5th-to-6th exon region in the carthlicoides accessions compared with Ldn (Fig. 2D).
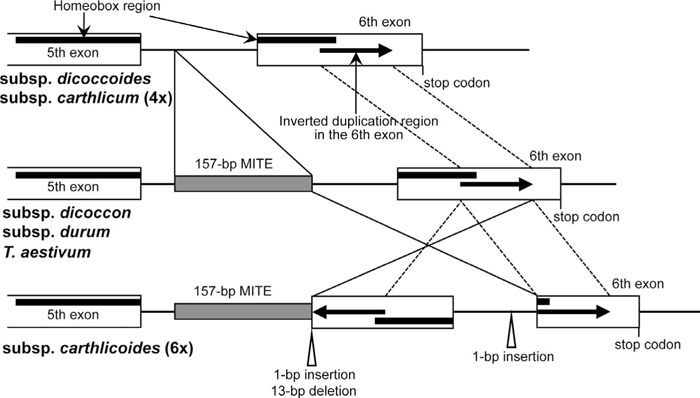
Schematic representation of the genomic structure of the Wknox1b 5th-to-6th exon region in tetraploid and hexaploid wheat subspecies.
Subspecies carthlicum has been defined morphologically by its long awn at an empty glume, and the four-awned phenotype of each spikelet is controlled by the tetraaristatus gene on 5AL (Haque et al., 2011). Therefore, Wknox1b on chromosome 4B is unlikely to be directly associated with subsp. carthlicum evolution. Nevertheless, our finding of the structural mutation at the Wknox1b locus of subsp. carthlicoides has implications for wheat speciation. Comparison of the genomic structure at the Wknox1b 5th-to-6th exon region among subspecies of tetraploid and hexaploid wheats strongly suggests that subsp. carthlicoides has not directly evolved from subsp. carthlicum, and is not a progenitor of other subspecies of hexaploid wheat (Fig. 3). It therefore appears likely that subsp. carthlicoides originated from other wheat hexaploids.
Subspecies carthlicum shares the same morphological characteristics as subsp. carthlicoides in spite of the differences in their genome constitution (Kuckuck, 1979; Haque et al., 2011). If a tight genetic relationship exists between subsp. carthlicum and subsp. carthlicoides, it is possible that subsp. carthlicum evolved from an interspecific cross between subsp. carthlicoides and another subspecies of T. turgidum based on comparison of their genomic structures in the Wknox1b 5th-to-6th exon region (Fig. 3). Interploidy hybridization between cultivated emmer wheat and subsp. carthlicoides was postulated to have resulted in the generation of subsp. carthlicum (Kuckuck, 1979). On the other hand, subsp. paleocholchicum is thought to have been generated by interploidy hybridization between wild emmer wheat and common wheat (Dvorak and Luo, 2001). The carthlicum allele for the Wknox1b 5th-to-6th exon region is the same as that in subsp. dicoccoides (Figs. 2 and 3), suggesting that subsp. carthlicum originated from interploidy hybridization between wild emmer wheat and subsp. carthlicoides, and that subsp. dicoccoides is a parent of subsp. carthlicum, as well as of subsp. paleocholchicum. To clarify the parental subspecies of tetraploid wheat involved in interploidy hybridization, a wide survey of cultivated emmer wheat accessions lacking the 157-bp MITE in the Wknox1b 5th intron would be required. Structural analysis of the Wknox1 loci is useful for understanding the complicated evolutionary process of allopolyploid wheat speciation.
Comparative expression analysis of the Wknox1 homoeologous copiesTo examine the effect of the inverted duplication in the 5th-to-6th exon region of Wknox1b on expression of homoeologous copies of Wknox1 in subsp. carthlicoides, RT-PCR was conducted with primer pairs specific to each homoeologous copy using RNA from seedlings having a SAM. Total RNA was extracted using Sepasol-RNA I Super G solution (Nacalai Tesque, Kyoto, Japan) from shoots of 7-day-old seedlings. First-strand cDNA was synthesized from DNase I-treated RNA samples with oligo-dT primers and ReverTra Ace reverse transcriptase (Toyobo, Osaka, Japan), and used for PCR. In RT-PCR of the three homoeologous Wknox1 transcripts, a common forward primer, 5’-GGAGGGTGGAGACGCAACTCAACT-3’, and the following three reverse primers, designed based on homoeolog-specific sequences in the 3’ untranslated region, were used: 5’-CACCGACCAAGGTCACCAGT-3’, 5’-CGCCGACCAAGGTGACCGGC-3’ and 5’-CCAAGGTCACCGGTAACGGT-3’ for Wknox1a, Wknox1b and Wknox1d, respectively. For amplification of the ubiquitin gene as a control, the gene-specific primer pair 5’-GCATGCAGATATTTGTGAA-3’ and 5’-GGAGCTTACTGGCCAC-3’ was used. For amplification of total Wknox1 transcripts, the following primer pair was used: 5’-TCTCCCACCCCCACTACTC-3’ and 5’-AGTTGCGTCTCCACCCTCCT-3’. Three homoeologous copies of Wknox1 were expressed in CS, and transcripts from both the Wknox1a and Wknox1b copies were observed in the subsp. carthlicum accessions (Fig. 4). In the two carthlicoides accessions, the Wknox1a and Wknox1d copies were expressed, but no Wknox1b transcript was observed.

RT-PCR analysis of transcript accumulation from Wknox1 and its three homoeologous copies. Total RNA was extracted from SAM-containing shoots of each accession. The ubiquitin gene was used as an internal control. All of the amplified DNA fragments were separated by electrophoresis through a 2% agarose gel and stained with ethidium bromide. The number of PCR cycles is shown at the right side of the electropherograms.
Thus, the inverted duplication in the Wknox1b 5th-to-6th exon region could suppress the expression of Wknox1b in subsp. carthlicoides (Fig. 4), suggesting that carthlicoides Wknox1b is a null allele. The functions of the Wknox1 orthologs are essential for SAM maintenance and plant development, and loss-of-function mutants in Arabidopsis and maize show lethal or extreme morphological defects due to disruption of the SAM (Long et al., 1996; Kerstetter et al., 1997). Although many gain-of-function mutation alleles of Wknox1 orthologs have been well studied in maize, tomato and barley (Smith et al., 1992; Müller et al., 1995; Chen et al., 1997), loss-of-function mutants are rarely found in diploid plant species. Therefore, the naturally occurring null mutation of Wknox1 is a unique characteristic of subsp. carthlicoides. The function of the two homoeologous copies, Wknox1a and Wknox1d, may complement the loss of Wknox1b function in subsp. carthlicoides, since allopolyploidy makes complementation possible in hexaploid wheat.
If the subsp. carthlicoides allele of Wknox1b is expressed, transcripts from the Wknox1b inverted-duplication region could form a stem-loop structure. Such transcripts could generate small interfering RNAs (Chapman and Carrington, 2007), possibly inhibiting expression of the homoeologous copies because of the high nucleotide similarity among the three homoeologous cDNAs of Wknox1 (Takumi et al., 2000). However, no transcript from the carthlicoides allele of Wknox1b was detected, and the Wknox1b inverted duplication appears to have no influence on expression of the other two homoeologous copies (Fig. 4). At present, we have no data that might explain the molecular mechanism of transcriptional suppression of the carthlicoides allele of Wknox1b. In common wheat, genetic and epigenetic modifications are reportedly associated with differential expression of the three homoeologous copies of developmentally important genes (Shitsukawa et al., 2007). Wknox1b suppression in subsp. carthlicoides is another interesting example for studies of the molecular mechanisms of functional differentiation among homoeologous copies in allopolyploid wheat.
Wheat seeds with KU and CGN accession numbers were supplied by the National BioResource Project-Wheat (Japan; www.nbrp.jp) and the Centre for Genetic Resources (The Netherlands), respectively. This work was supported by grants from the Ministry of Education, Culture, Sports, Science and Technology of Japan (Grant-in-Aid for Scientific Research (B) Nos. 21380005 and 25292008).