INTRODUCTION
Insulin-like growth factor 2 (IGF2) mRNA-binding proteins (IMPs), including IMP1, IMP2 and IMP3, belong to a family of RNA-binding proteins (RBPs). They are characterized by a conserved RNA-binding domain that consists of two RNA recognition motifs (RRMs) and four hnRNP K homology (KH) domains (RNA-binding motifs) (Deshler et al., 1998; Hansen et al., 2004; Yisraeli, 2005). IMPs are involved in posttranscriptional processes including localization, stability and translational control of their target mRNAs (Yaniv and Yisraeli, 2002) to regulate several aspects of cell function, such as the formation of mesoderm during early stages of oocyte development (Deshler et al., 1997; Havin et al., 1998), the migration of neural crest cells during maturation of the nervous system (Ross et al., 1997; Eom et al., 2003; Vikesaa et al., 2006), and translational adaptation during cellular stress (Stöhr et al., 2006).
As IGF2-binding proteins, IMPs have been reported to be critical in growth and development (Hansen et al., 2004; Yisraeli, 2005). The IGFs are mitogens that play a vital role in regulating cell proliferation, differentiation and apoptosis, and function as key regulators during embryonic and fetal growth (Yu and Rohan, 2000). IMP1−/− mice were found to be 40% smaller than the wild-type (Hansen et al., 2004), and this dwarf phenotype of mice in response to IMP down-regulation is due to the correspondingly reduced expression of the IGF2 gene (Nezer et al., 1999; Christiansen et al., 2009). IMP3 gene knockdown decreased IGF2 protein expression by inhibiting the translation of IGF2 mRNA, which eventually led to the suppression of cell proliferation, while subsequent addition of recombinant IGF2 polypeptide to the cells could restore cell proliferation rates to normal (Liao et al., 2005). Thus, IMPs are considered to be closely related to growth through their regulation of IGF2 protein synthesis (Dai et al., 2011). Besides the effect on the IGF system, IMPs are related to transcripts involved in cell cycle control, fatty acid synthesis and metabolism, and cell signaling, which may also influence development and growth (Hansen et al., 2004).
Bivalves are globally distributed species and belong to the second-largest phylum of the animal kingdom, Mollusca (Kocot et al., 2011). As one of the most important biological groups with essential roles in energy flow and as a food resource, many bivalve species have been cultured widely, and growth improvement is one of the main focuses of genetic breeding. To date, several genes and loci related to bivalve growth have been identified, including those in the IGF-related system (Guo et al., 2012; Cong et al., 2013; Feng et al., 2014). For example, single-nucleotide polymorphisms (SNPs) associated with growth traits were found in the IGF-binding protein gene of Patinopecten yessoensis (Feng et al., 2014), and an insulin receptor-related receptor involved in insulin-like effects on growth was detected in Crassostrea gigas (Gricourt et al., 2003). For IMPs, on the other hand, there has been no report on these IGF-influencing growth factors in any bivalve or mollusk species.
In the present study, we cloned the IMP gene (PyIMP) from the Yesso scallop, P. yessoensis, which is one of the most important cultured marine bivalves in the north of China. Its genomic organization, protein primary structure, and expression in adult tissues and embryo/larva stages were analyzed. Meanwhile, a SNP significantly associated with scallop growth as well as PyIMP expression was identified in this gene. PyIMP represents the first mRNA-binding protein characterized in mollusks, and our results will facilitate a better understanding of IMP function in scallops.
MATERIALS AND METHODS
Scallop collection
Scallops used in this study were all collected from Zhangzidao Fishery Group Co., Dalian, China. Two Yesso scallop populations were used for gene mutation screening and genotyping, and each population was established with more than 2,000 sexually mature scallops through artificial fertilization. For the first population (population I), ten Yesso scallops (13 months old) with obvious differences in size and weight were collected for mutation scanning. For the second population (population II), 119 individuals (10 months old) were randomly collected for locus verification and marker development. Five growth traits, shell length (SL), shell height (SH), body weight (BW), soft tissue weight (STW) and striated muscle weight (SMW), were measured. The striated muscle of all the sampled scallops was dissected, frozen in liquid nitrogen and stored at −80 ℃. Embryos and larvae of Yesso scallop including the fertilized eggs, blastulae, gastrulae, trochophore and D-shaped larvae were collected. For each developmental stage, three parallel samples were collected with at least 500 embryonic/larval individuals for each sample. All embryonic/larval samples were temporarily stored in RNAwait (Solarbio, Beijing, China) and preserved at −80 ℃.
RNA isolation and cDNA synthesis
Total RNA was extracted from the striated muscles using the guanidinium isothiocyanate method (Hu et al., 2006), and then digested with DNase I (TaKaRa, Shiga, Japan) to remove residual DNA. First-stand cDNA was synthesized by M-MLV Reverse Transcriptase (Invitrogen, CA, USA). A control reaction without reverse transcriptase was performed for precluding any DNA contamination. In brief, the reaction was performed in a 20-μl volume containing 2 μg DNase I-treated total RNA as template, 0.5 μM Oligo (dT)18 (TaKaRa) as primer, 1× reaction buffer, 20 U RNase inhibitor (Invitrogen), 1 mM dNTPs (Invitrogen) and 200 U reverse transcriptase. All the components were added in the indicated order on ice. cDNAs were synthesized by incubating the reaction mixture at 42 ℃ for 90 min, and the reaction was terminated by heating at 70 ℃ for 5 min. The cDNA was diluted 1:30 and stored at −20 ℃.
Full-length cDNA cloning for PyIMP
An IMP gene fragment of 2,490 bp was obtained from the transcriptome data of Yesso scallop (Hou et al., 2011). 3’ and 5’ rapid amplification of cDNA ends (RACE) were performed using a SMART RACE cDNA Amplification Kit (Clontech, CA, USA). The gene-specific primers IMP-3f and IMP-5r (Supplementary Table S1) were designed for 3’ and 5’ RACE, respectively. The RACE fragments were ligated into the pMD18-T vector (TaKaRa), and recombinant plasmids with inserts from both 3’ and 5’ RACE were sequenced by Sangon Biotech (Shanghai, China). The full-length cDNA sequence was determined by assembling the sequences of the 3’ and 5’ RACE products.
Sequence analysis
The assembled cDNA sequence was analyzed with the BLAST (blastx) algorithm at the National Center for Biotechnology Information database (http://www.ncbi.nlm.nih.gov/blast/) for evaluating similarity to known genes. ORF (open reading frame) finder (http://www.ncbi.nlm.nih.gov/gorf/gorf.html) was used to predict amino acid sequences. The deduced protein sequence was then analyzed using the blastp algorithm for similarity analysis to known genes for annotation. Conserved domains were predicted by the simple modular architecture research tool (SMART) (http://smart.embl-heidelberg.de/). Putative isoelectric point (pI) and molecular mass were computed using the Compute pI/Mw tool (http://web.expasy.org/compute_pi/). The full-length cDNA sequence of PyIMP was aligned to the Yesso scallop genome database (GenBank accession no. SAMN03654043) using GMAP (http://research-pub.gene.com/gmap/) to search for the genomic sequence of PyIMP. Gene structure was then determined by comparing the genomic and cDNA sequences.
The IMPs from Caenorhabditis elegans, Drosophila melanogaster, Ciona intestinalis, Branchiostoma floridae and Homo sapiens were aligned using the Clustal Omega multiple alignment program (http://www.ebi.ac.uk/Tools/msa/clustalo/) (Supplementary Table S2). Multiple alignment of the IMPs was performed using the GeneDoc multiple alignment editor (http://www.nrbsc.org/gfx/genedoc/index.html). The neighbor-joining (NJ) algorithm in the MEGA 6.0 software (http://www.megasoftware.net/) was used to construct an unrooted phylogenetic tree based on the amino acid sequences of IMPs. Aligned sequences were bootstrapped 1000 times to derive a confidence value for phylogenetic analysis.
PyIMP expression analysis
Adult tissues from six Yesso scallops and the embryos/larvae collected were used for PyIMP expression analysis. First-strand cDNA was synthesized from 2 μg total RNA for each sample using Oligo (dT)18 and M-MLV reverse transcriptase (Invitrogen) in a total volume of 25 μl. The expression levels of PyIMP in adult tissues and developmental stages of Yesso scallop were analyzed using real-time quantitative reverse transcription PCR (qRT-PCR). The specific primers IMP-f1 and IMP-r1 (Supplementary Table S1) were designed for amplification of PyIMP cDNA. Genes encoding DEAD-box RNA helicase-like protein, ubiquitin and 60S ribosomal protein L16 were selected as reference genes for tissue samples; cytochrome b, cytochrome c and histone H3.3 were used as reference genes for embryo/larva samples (Feng et al., 2013).
The qRT-PCR amplification was performed as follows: initial denaturation at 95 ℃ for 10 min, followed by 40 cycles of 95 ℃ for 15 s and 60 ℃ for 1 min. For each PCR, three technical replicates were performed. To exclude the possibility that non-specific products were present in the PCR products, a dissociation analysis was performed by subjecting the samples to a gradual decrease in temperature (from 95 ℃ to 60 ℃). The PCR products of PyIMP and the reference genes were purified and sequenced by Sangon Biotech (Shanghai, China) for specificity verification. The results of qRT-PCR were analyzed using Real-time PCR Miner (http://www.miner.ewindup.info/). The geometric means of the values generated with the three reference genes were calculated for both tissue and embryo/larva samples for normalization (Feng et al., 2013). The expression levels of PyIMP among adult tissues and developmental stages were compared and analyzed using one-way ANOVA followed by Duncan’s new multiple range test.
Mutation scanning, genotyping and association analysis
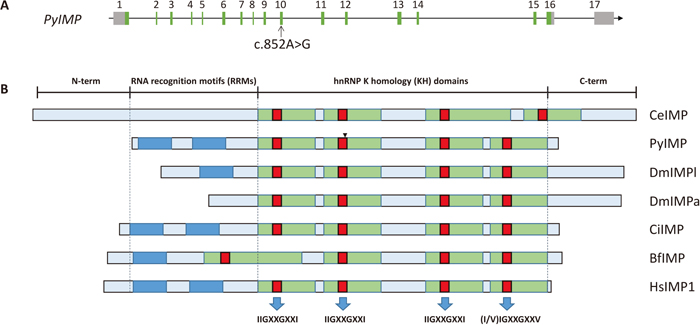
Ten adult Yesso scallops from population I with obvious differences in size and weight were used for mutation scanning. Genomic DNA was extracted from the striated muscle by the phenol/chloroform extraction method (Sambrook and Russell, 2001). Primers IMP-f2/r2, IMP-f3/r3, IMP-f3/r3, IMP-f4/r4, IMP-f5/r5, IMP-f6/r6, IMP-f7/r7, IMP-f8/r8 and IMP-f9/r9 (Supplementary Table S1) were used to amplify the exon sequences of PyIMP in the ten scallops. PCR was performed in a 50-μl volume containing 1× Advantage2 PCR buffer, 0.2 mM of each dNTP (Invitrogen), 0.2 μM of each primer, 50 ng genomic DNA and 1 μl 50× Advantage2 Polymerase Mix (Clontech). The amplification was performed as follows: 95 ℃ for 5 min; 30 cycles of 95 ℃ for 30 s, 62 ℃ for 30 s and 72 ℃ for 60 s; and a final extension at 72 ℃ for 5 min. The PCR fragments were purified and sequenced by Sangon Biotech. A SNP (c.852A>G) located at 852 bp downstream from the start codon was found in the tenth exon of PyIMP (Fig. 1).
SNP c.852A>G was then genotyped with the high-resolution melting (HRM) method in 119 scallops from population II for marker development (Guo et al., 2012). Primers IMPsf1 and IMPsr1, and probe IMPpb1, were designed for c.852A>G genotyping (Supplementary Table S1). PCR was performed in a 10-μl reaction containing 1× PCR buffer, 1.5 mM MgCl2, 0.5 U Taq DNA polymerase (TaKaRa), 0.2 mM of each dNTP (Invitrogen), 0.1 μM forward primer, 0.5 μM reverse primer, 1× LCGreen Plus (Idaho Technology, UT, USA) and 20 ng genomic DNA. The amplification was performed as follows: 95 ℃ for 5 min; 60 cycles of 95 ℃ for 40 s, 63 ℃ for 40 s and 72 ℃ for 40 s; and a final extension at 72 ℃ for 5 min. The probe IMPpb1 (3 μM) was added to each successfully amplified PCR product, and the mixture was then denatured at 95 ℃ for 10 min and slowly cooled to 40 ℃. HRM was immediately performed using a Light-Scanner instrument (Idaho Technology) with continuous melting curve acquisition (10 acquisitions per ℃) over a 0.1 ℃/s ramp from 40 to 95 ℃. The data were retrieved and analyzed using the Light Scanner software (Idaho Technology), followed by manual curating of the genotype results. The HRM analysis curves for homozygotes and heterozygotes are shown in Supplementary Fig. S1.
The scallops were grouped according to their genotypes. A chi-squared test was used to examine Hardy-Weinberg equilibrium (HWE). Values for each trait (presented as mean ± S.D.) among different genotypes were compared and analyzed with one-way ANOVA followed by Duncan’s new multiple range test. The association between growth traits and SNP c.852A>G was analyzed using PLINK (Purcell et al., 2007). P values of < 0.05 were considered statistically significant.
Association analysis of PyIMP expression and c.852A>G genotype
To compare PyIMP expression levels among scallops with different genotypes, RNA-seq data (X. Li et al., unpublished) from 24 Yesso scallops (20 months old) collected from a population bred by mass spawning were analyzed. PyIMP expression and c.852A>G genotype of each individual were extracted from these data. The expression levels of PyIMP and the growth traits among different genotypes were then compared with one-way ANOVA followed by Duncan’s new multiple range test.
RESULTS AND DISCUSSION
Characterization of PyIMP gene sequence
From the transcriptome data of Yesso scallop (Hou et al., 2011), a 2,490-bp cDNA fragment of the PyIMP gene was obtained. Next, 509-bp and 551-bp cDNA sequences were obtained by 5’ and 3’ RACE, respectively. After assembling these sequences we obtained the full-length PyIMP cDNA sequence, which contained 3,293 bp, with an open reading frame of 1,776 bp, and encoded 592 amino acids. The predicted molecular mass of the PyIMP protein was 64.9 kDa, with a predicted isoelectric point (pI) of 8.88. Comparison of the cDNA and genomic DNA sequences of this gene showed that the complete DNA sequence of PyIMP was 22,875 bp, consisting of seventeen exons and sixteen introns. Inspection of the exon-intron boundaries indicated that they all conformed to the GT-AG rule (Sharp, 1981). The structure of the PyIMP gene is shown in Fig. 1. The genomic and cDNA sequences of PyIMP have been deposited in the GenBank database under accession numbers KP311301 and KP311302, respectively.
Investigating the genome databases of P. yessoensis, C. gigas (GenBank accession no. AFTI00000000) and Chlamys farreri (unpublished) identified only one IMP gene in each of these species, consistent with the results obtained in other invertebrate organisms such as C. elegans and D. melanogaster, which possess one IMP copy in the genome (C. elegans Sequencing Consortium, 1998; Adams et al., 2000). IMP has expanded notably through evolution, however, with three members, IMP1, IMP2 and IMP3, in vertebrates (Zhang et al., 1999a; Yaniv and Yisraeli, 2002; Le et al., 2012). Despite possessing only one IMP gene, eleven splice variant transcripts of IMP were observed in D. melanogaster, and such a phenomenon appears to be common in Arthropoda (four splice variants of the IMP gene in both Apis mellifera and Bombyx mori). Gene duplication and alternative splicing are the two main processes responsible for expanding protein functional diversity (Keren et al., 2010; Le et al., 2012). In the current study, we found no IMP splicing variants in the scallop transcriptome, which was constructed using RNA-seq data from ten different adult tissues and seven embryo/larva stages. We speculate that the bivalve IMP gene, which lacks duplication or splice variants, represents a relatively ancient form of IMP.
Alignment and phylogeny analysis of IMPs
Multiple sequence alignment analysis revealed that the amino acid (aa) sequence of PyIMP exhibits characters typical of IMPs, including two RRMs and four KH domains (Fig. 1, Fig. 2). The RRM regions were located at aa 3–69 and 79–145 of PyIMP protein. Four KH domains were found at aa 181–253, 262–335, 406–486 and 497–571, with highly conserved IIGXXGXXI motifs (IIGKQGQTI, IIGKQGNVI, IIGSKGANI and IIGKGGQNV, respectively) (Fig. 2). To date, the two-RRM and four-KH structure has also been found in the IMPs of vertebrate species, such as human, mouse and zebrafish (Zhang et al., 1999a; Le et al., 2012; Gaynes et al., 2015). Although all the IMPs identified to date contain four KHs, the number of RRMs in IMPs is diverse in invertebrate organisms. For example, C. intestinalis and bivalve IMP both possess two RRM domains (Christiansen et al., 2009), but the homologs from B. floridae (GenBank accession number XP_002600650.1) and D. melanogaster (GenBank accession number NP_001245616.1) have only one RRM, and the RRM is missing altogether in C. elegans IMP (GenBank accession number NP_001022695.1). Furthermore, disappearance of RRM by alternative translational initiation and alternative splicing was also found in mammalian (Le et al., 2012) and D. melanogaster IMPs (GenBank accession number NP_511111.1), respectively. It has been revealed that the mRNA-binding functions of IMP are mainly carried out by the KH domains (Farina et al., 2003), although RRM was previously considered to participate in this process (Nielsen et al., 1999). In addition, deletion of the two RRMs from chicken IMP demonstrated that the C-terminal KHs were sufficient for RNA binding in vitro (Nielsen et al., 2002; Farina et al., 2003), implying that the RRMs in IMP are not necessary for RNA binding. Meanwhile, in mammals, the canonical 66-kDa isoform with two RRMs and four KHs, and a 58-kDa isoform lacking the RRM1 domain, showed tissue differences in expression patterns, suggesting functional differences between these isoforms (Le et al., 2012). The diversity of RRM number in invertebrate IMP also implies functional variation of IMP among species, and even the dispensability of this domain for IMP function in some species. Here, in the three bivalve species P. yessoensis, C. gigas and C. farreri, the IMPs contained the full repertoire of two RRMs and four KH domains, indicating that structurally complete IMP genes already existed in mollusks, while RRM domains were lost in several species during eukaryotic evolution or lost in certain spliced transcripts. The diversity of RRM number among invertebrate IMPs and among IMP isoforms implies relaxed selection on this domain, which could provide IMP with plasticity for its binding targets and binding affinity, and thereby generate functionally new products and create phenotypic complexity.

A phylogenetic tree was constructed to determine the evolutionary position of PyIMP using the known IMP proteins from mammals, amphibians, fish, birds, amphioxus, ascidians, insects and mollusks (Fig. 3). In vertebrates, the IMP proteins were categorized into three subfamilies, IMP1, IMP2 and IMP3. The single IMP of C. elegans was in the most peripheral of the phylogenetic clusters, and those of scallops and oyster were grouped together and generated clades that ulteriorly formed into larger clusters. According to previous studies that have focused on vertebrate IMP family genes, the IMP subfamilies divided by phylogenetic analysis are likely to have separate functions (Nielsen et al., 2000; Yisraeli, 2005; Le et al., 2012). For instance, IMP1 is orthologous to chicken ZBP-1 and mouse CRD-BP, which have been implicated in sorting β-actin mRNA and stabilizing c-myc mRNA, respectively; IMP2 was identified as an autoantigen in hepatocellular carcinoma; IMP3 was identical to the KOC protein, which is overexpressed in pancreatic cancer, and orthologous to Xenopus Vg1-RBP, which has been implicated in mRNA trafficking (Deshler et al., 1997; Mueller-Pillasch et al., 1997; Doyle et al., 1998; Havin et al., 1998; Nielsen et al., 1999; Brants et al., 2004). After the protochordates, obvious gene expansion arose due to whole-genome duplications, gene duplications and retrotranspositions, accompanied by gene functional differentiation and specialization (Panopoulou and Poustka, 2005). On this basis, as a potential ancient form of IMPs, further functional analysis is needed for PyIMP to consummate the functional evolution of this family.

Expression of PyIMP in embryos/larvae and adult tissues of Yesso scallops was analyzed using qRT-PCR. Among the five developmental stages tested, significantly more PyIMP transcripts were detected in gastrula and trochophore larvae than in other stages (Fig. 4A). By the gastrula stage, the expression of PyIMP had increased sharply compared to that in the fertilized eggs and blastulae. After the trochophore larva stage, expression was significantly down-regulated in D-shaped larvae, where the transcription level of PyIMP was the lowest among all the stages tested (Fig. 4A). It has been reported that Xenopus IMP3 directs the localization of mRNAs that mediate the signaling of gastrula stage to the vegetal pole of the oocyte (Deshler et al., 1997; Kwon et al., 2002; Holland and Onai, 2012), and chicken IMP1 appears to direct mRNA localization to the leading edge of embryonic fibroblasts (Farina et al., 2003). These data suggest that IMPs play a pivotal role in modulating both cytoskeletal polarization and actin-driven cell migration in early embryonic developmental stages (Bell et al., 2013). Significantly more PyIMP transcripts were detected in gastrulae and trochophore larvae, implying that PyIMP participates in aspects of early embryonic development, such as gastrula organization, in scallop.
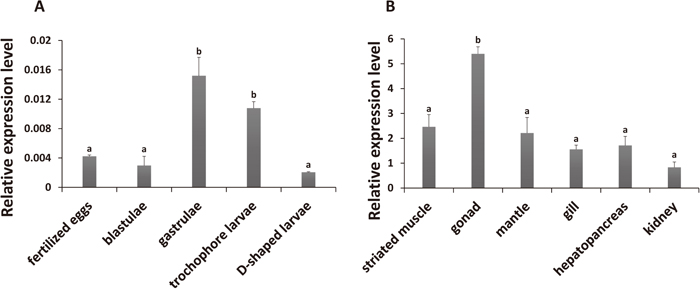
PyIMP mRNAs were also detected in all adult tissues sampled (Fig. 4B, Supplementary Fig. S2). In human, IMP1 transcripts were present in adult kidney, testis and ovary; IMP3 expression was detected in brain, testis and ovary; whereas IMP2 transcripts were more widespread but with low expression levels (Mueller-Pillasch et al., 1997; Nielsen et al., 1999; Zhang et al., 1999b). Compared to the universal expression of the PyIMP gene, the different expression patterns among IMP genes in human indicates the functional specialization of IMPs during evolution. A significantly higher level of PyIMP expression was found in gonad than in the other tissues (Fig. 4B). As the scallops used for tissue expression analysis in this study were gonadal immature, we further analyzed PyIMP expression in mature male and female gonads (Supplementary Fig. S2), using transcriptome data generated by RNA-seq (SRS935829 and SRX1447196, respectively). No significant difference in PyIMP expression was detected between the male and female gonads (P = 0.155). In mouse, IMPs display a burst of expression in male and female gonadal cells at embryonic day 12.5 (E12.5), when morphological differences between ovaries and testis become apparent, and decline toward birth (Nielsen et al., 1999). Phosphorylation of Xenopus IMP3 by MAPKs was suggested to modulate the release of Vg1 mRNA from mRNPs localized to the vegetal cortex during meiotic maturation (Git et al., 2009). Significantly more abundant PyIMP transcripts in gonad implies that the gene is involved in gametogenesis in scallop.
Association between a PyIMP SNP and scallop growth
After investigating the sequence variants in PyIMP, a synonymous SNP named c.852A>G was found in the tenth exon (Fig. 1). We then analyzed the trait values of 119 scallops from a cultured population with respect to the genotype of this locus. Significant associations were detected between SNP c.852A>G and all the traits tested, namely SL (P = 0.029), SH (0.047), BW (0.035), STW (0.037) and SMW (0.041). For all of the growth traits measured, the highest and lowest trait values were found in scallops with AA and GG genotypes, respectively, while AG type individuals showed intermediate values (Table 1). A significant difference (P < 0.05) in trait values was present between genotypes AA and GG for SL, SH, BW, STW and SMW, using ANOVA followed by Duncan’s new multiple range test. To further understand the association, we then analyzed an RNA-seq dataset generated from 24 scallops (X. Li et al., unpublished) to investigate the associations between SNP c.852A>G genotype and PyIMP expression. The results indicated that PyIMP expression in AA type scallops was significantly higher than in GG scallops (Fig. 5). Moreover, in these scallops, AA genotype individuals had higher growth trait values than GG type (Supplementary Table S3), consistent with the results obtained in the 119 individuals of the previous population. Therefore, scallops with AA genotype at SNP c.852A>G had higher growth trait values and a higher PyIMP expression level, suggesting that PyIMP has a positive effect on the growth of Yesso scallop; this is consistent with an analysis in mice, where IMP1−/− individuals were smaller than wild-type ones (Hansen et al., 2004). Further investigation of PyIMP expression with a larger population size and functional assays will provide more convincing evidence.
Table 1. Growth traits of Yesso scallops with different genotypes at SNP c.852A > G
| Genotype | N/F | PHWE | SL | SH | BW | STW | SMW |
|---|
| GG | 29/24.2 | 0.89 | 36.36 ± 5.66a | 37.67 ± 5.66a | 6.09 ± 3.0a | 3.46 ± 1.73a | 0.58 ± 0.31a |
| AA | 35/29.2 | 39.79 ± 7.10b | 40.82 ± 7.22b | 7.88 ± 3.90b | 4.50 ± 2.41b | 0.82 ± 0.74b |
| AG | 55/45.8 | 37.43 ± 5.93ab | 38.79 ± 5.92ab | 6.69 ± 3.03ab | 3.81 ± 1.78ab | 0.62 ± 0.29ab |
N, number of scallops; F, genotype frequency (%); PHWE, P value for Hardy-Weinberg equilibrium test; SL, shell length (mm); SH, shell height (mm); BW, body weight (g); STW, soft tissue weight (g); SMW, striated muscle weight (g). Growth trait values are given as the mean ± standard deviation. Values with different superscripts within each column are significantly different (P < 0.05).
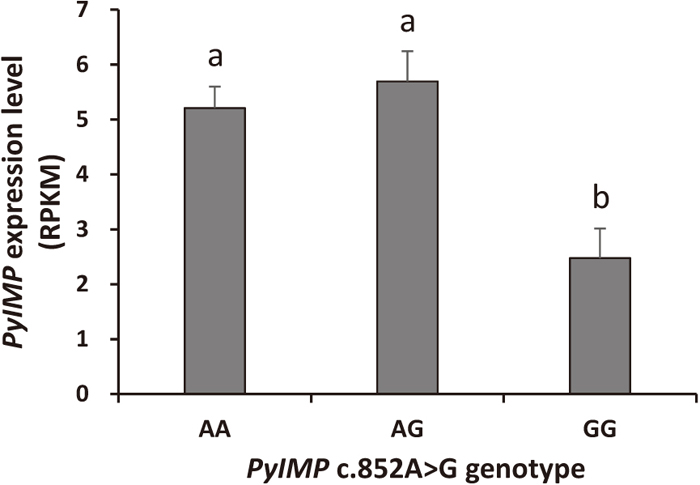
Previous studies have shown that IMPs play important roles in growth and development (Nielsen et al., 1999; Brants et al., 2004; Hansen et al., 2004). IMP was able to bind to the mRNA of IGF2 and enhance its stability, and cytoplasmic IMP proteins could control IGF2 biosynthesis during late mammalian development (Nielsen et al., 1999; Nielsen et al., 2002; Szarvas et al., 2012). In bivalves, the IGF ligands (IGF1 and IGF2), IGF receptors and IGF-binding proteins are known to affect growth (Kellner-Cousin et al., 1994; Hamano and Awaji, 2002; Cong et al., 2013; Feng et al., 2014). Here, we found a SNP in PyIMP that was significantly associated with scallop growth, indicating the possible growth-regulating function of this gene, which was further supported by the association of PyIMP expression and the genotypes at this locus. SNPc.852A>G is a synonymous mutation that does not change the amino acid at this locus. It has been shown that synonymous mutations can be used to encode additional information to affect the speed or accuracy of mRNA translation, mRNA folding, mRNA splicing and protein folding through translational pausing (Supek et al., 2014). Here, PyIMP expression levels in scallops genotyped as AA were significantly higher than those in scallops genotyped as GG, which implies that SNP c.852A>G is related to the post-transcriptional regulation of the gene.
In conclusion, we cloned an IMP gene in Yesso scallop, and showed that there is only one IMP gene in this bivalve genome. The gene sequence and its ontogenic and adult tissue expression were also analyzed. A synonymous SNP that significantly associated with scallop growth traits was identified in this gene. PyIMP represents the first mRNA-binding protein gene characterized in mollusks, which will assist in developing a better understanding of the evolution of mRNA-binding proteins. Further in-depth studies on the expression regulation of PyIMP and its relationship with scallop growth should help to unveil the biological functions of this gene.






