2018 Volume 93 Issue 4 Pages 125-133
2018 Volume 93 Issue 4 Pages 125-133
Recently published structural and functional analyses of the CMG complex have provided insight into the mechanism of its DNA helicase function and into the distinct roles of its central six component proteins MCM2-MCM7 (MCM2-7). To activate CMG helicase, the two protein kinases CDK and DDK, as well as MCM10, are required. In addition to the initiation of DNA replication, MCM function must be regulated at the DNA replication steps of elongation and termination. Polyubiquitylation of MCM7 is involved in terminating MCM function. Reinitiation of DNA replication in a single cell cycle, which is prevented mainly by CDK, is understood at the molecular level. MCM2-7 gene expression is regulated during cellular aging and the cell cycle, and the expression depends on oxygen concentration. These regulatory processes have been described recently. Genomic structural alteration, which is an essential element in cancer progression, is mainly generated by disruptions of DNA replication fork structures. A point mutation in MCM4 that disturbs MCM2-7 function results in genomic instability, leading to the generation of cancer cells. In this review, I focus on the following points: 1) function of the MCM2-7 complex, 2) activation of MCM2-7 helicase, 3) regulation of MCM2-7 function, 4) MCM2-7 expression, and 5) the role of MCM mutation in cancer progression.
MCMs were initially identified as genes that play essential roles in the maintenance of extrachromosomal DNA in Saccharomyces cerevisiae. In eukaryotic cells, MCM2-MCM7 (MCM2-7) form a heterohexameric complex that functions as a replicative DNA helicase to unwind the DNA duplex template during DNA replication. A recently published structural analysis of the S. cerevisiae MCM2-7 complex indicated that conserved loop structures are located inside the ring structure of the complex (Li et al., 2015). These loop structures may interact with unwound single-stranded DNA to allow the MCM complex to translocate along the DNA (Brewster et al., 2008; Seo and Kang, 2018). Primary structures of human MCM2-7 show that a conserved MCM box is in the central region (Fig. 1). Amino acid sequences required for nuclear localization are present in the amino-terminal region of MCM2 and the carboxyl-terminal region of MCM3. The biochemical stability of MCM complexes suggests that newly synthesized MCM2, 4, 6 and 7 proteins form a heterotetrameric complex that translocates into the nucleus, whereas MCM3 and 5 form another complex that also translocates into the nucleus. These two complexes are assembled into the heterohexameric MCM2-7 complex at the DNA replication origin, with the assistance of CDT1 and CDC6, where the origin recognition complex (ORC) has been assembled (Forsburg, 2004; Masai et al., 2010). Distinct nuclear export signals are present in the central region of MCM3. In higher eukaryotic cells, nuclear export of the MCM complex after it exerts its function has not been reported. However, recent data suggest that MCM complexes are shuttled between the cytoplasm and nucleus in interphase cells under the influence of the nuclear export signals in MCM3 (Chuang et al., 2012), similar to their behavior in S. cerevisiae.

Characteristics of the primary structures of human MCM2-7 proteins. The MCM box (dark gray) in the central region where the Walker A and B motifs are located, NLS (nuclear localization signal), and Zn-finger motifs (Zn) are indicated. A histone-binding domain (HBD) in MCM2 is also indicated. Positively charged amino acids in MCM4, 6 and 7 that may directly be involved in interaction with single-stranded DNA during DNA helicase action are indicated by arrows. K600 in MCM4, K486 in MCM6 and K471 in MCM7 correspond to K602 in MCM4, R478 in MCM6 and K471 in MCM7 in Drosophila, respectively. Numbers indicate amino acid positions and, to the right, the length of each polypeptide.
It was first suggested that the MCM complex has DNA helicase activity based on the presence of ATP-binding motifs (Koonin, 1993); this activity was initially detected in isolated human and mouse MCM4/6/7 hexameric complexes in vitro (Ishimi, 1997). The DNA helicase activity of the MCM complex was confirmed for a single MCM from Archaea. Later, MCM4/7 from S. cerevisiae (Kanter et al., 2008) and MCM6 from plants (Tan et al., 2010) were shown to possess DNA helicase activity. Commonly, these MCMs form hexameric complexes and exhibit DNA helicase activity. It has been reported that the MCM2-7 complex from S. cerevisiae exhibits the DNA helicase activity under specific reaction conditions (Bochman and Schwacha, 2008). The MCM2-7 hexamer complexes with CDC45 and the GINS complex to form the CMG complex. CDC45 interacts with MCM2, and the GINS complex interacts with MCM3/5 (Ali et al., 2016) (Fig. 2). The CMG complex exhibits distinct and processive DNA helicase activity in vitro (Kang et al., 2012). Several lines of evidence support the notion that the CMG complex functions as the replicative DNA helicase in vivo.

Structure of the CMG complex bound to DNA. The CMG complex bound to DNA with fork-like structure is shown based on a cryo-electron microscopic analysis of the Drosophila CMG complex (Ali et al., 2016).
Cryo-electron microscopic analysis of the Drosophila CMG complex bound to DNA suggested that MCM4, 6 and 7, which are adjacently located in the MCM2-7 hexamer, directly contact DNA through specific amino acids in the central region (Ali et al., 2016). A set of positively charged residues (Mcm7 K471, Mcm4 K602 and Mcm6 R478) on the PS1 (pre-sensor 1) hairpins (Lyer et al., 2004; Brewster et al., 2008) contact and spiral around the nucleic acid in the Drosophila CMG complex. In the amino-terminal regions of MCM subunits, sets of two basic amino acids are conserved from human to Archaea, for example R291 and K353 in MCM4, R143 and R207 in MCM6, and R169 and K236 in MCM7 in human, while only one of the two conserved residues is present in the amino-terminal region of MCM2, MCM3 and MCM5 (Froelich et al., 2014). Conversion of these two conserved basic amino acids in either Mcm4, 6 or 7 in S. cerevisiae to aspartic acid did not affect cell growth, but combinations of the changes in Mcm4/6, Mcm4/7 or Mcm6/7 did not support DNA replication in vitro and resulted in loss of viability (Fig. 3). A mutant MCM complex from Archaea in which the conserved basic amino acids were changed to alanine lost its single-stranded DNA-binding activity (Froelich et al., 2014). Collectively, these findings suggest that MCM4/6/7 is directly involved in translocation along single-stranded DNA in the MCM2-7 complex.

Effect of mutations at basic amino acids of Mcm4, 6 and 7 on cell growth in S. cerevisiae. Two conserved basic amino acids in the amino-terminal region of Mcm4, 6 and 7 proteins were mutated to aspartic acid in S. cerevisiae (Froelich et al., 2014). The effect of mutations in single MCMs and double MCMs on cell growth is indicated.
A region in which acidic amino acids are clustered is present in the amino-terminal region of MCM2 (Fig. 4A). This region can bind to the central region (amino acid residues 26 - 67) of histone H3 (Ishimi et al., 1998). Mouse Mcm2 can assemble a nucleosome-like structure with purified histones in vitro via this histone-binding activity (Ishimi et al., 2001). Nucleosomal histones loosely associated with template DNA during SV40 chromosome replication in vitro are reassembled onto replicated DNA (Ishimi et al., 1991). MCM2 may be involved in transferring parental histone H3/H4 to newly replicated DNA in eukaryotic cells (Fig. 4B). Several findings support the notion that the amino-terminal region of MCM2 is required for reassembly of parental nucleosomal histones onto replicated DNA during cellular DNA replication (Foltman et al., 2013; Huang et al., 2015). Data suggest that both MCM2 in the CMG complex and FACT are involved in this function. MCM2 also plays a role in the assembly of newly synthesized histones onto replicated DNA. Thus, individual MCM2-7 proteins in the CMG complex may play distinct roles in chromosomal DNA replication.

Amino acid sequence in the amino-terminal region of MCM2. (A) The amino acid sequence in the amino-terminal region of human MCM2 is shown, with the histone-binding domain underlined. Tyrosine residues at 81 and 90 play an essential role in histone binding (Foltman et al., 2013). Three SSP sites that may be sequentially phosphorylated with CDK and DDK are underlined. (B) Postulated location of the MCM2-7 complex in the replication fork. MCM2 exerts the specific function of transferring histone H3/H4 on the parental DNA to the newly synthesized DNA.
Two protein kinases, CDK and DDK, are required for the initiation of DNA replication. It has been shown that CDK phosphorylates Sld2 and Sld3. Phosphorylated Sld2 complexed with GINS and DNA polymerase ε binds to a phospho-acceptor domain on Dpb11 in S. cerevisiae (Araki, 2011). Phosphorylated Sld3 complexed with Cdc45 binds to another phospho-acceptor domain on Dpb11. Three Mcm proteins, Mcm2, Mcm4 and Mcm6, in the Mcm2-7 complex pre-loaded onto the replication origin in the presence of the ORC, Cdc6 and Cdt1 are phosphorylated by DDK. In S. cerevisiae, the amino-terminal region of Mcm4 plays an inhibitory role in Mcm function (Sheu and Stillman, 2010), and DDK phosphorylates several amino acids to release this inhibitory activity (Fig. 5). Sld3 complexed with Dpb11 binds to Mcm4 and Mcm6 phosphorylated with DDK (Deegan et al., 2016), resulting in the assembly of a large protein complex at the origin of DNA replication (Fig. 6). TopBP1 (Tanaka et al., 2013) and Treslin (Kumagai et al., 2010) in higher eukaryotic cells were shown to be counterparts of Dpb11 and Sld3, respectively, and the amino-terminal region of RecQL4 has partial sequence homology to Sld2 (Matsuno et al., 2006). It has been shown that efficient function of Sld3 requires Sld7 (Tanaka et al., 2011). The carboxyl-terminal region of MTBP, the partner of Treslin, exhibits homology with Sld7 (Kumagai and Dunphy, 2017).
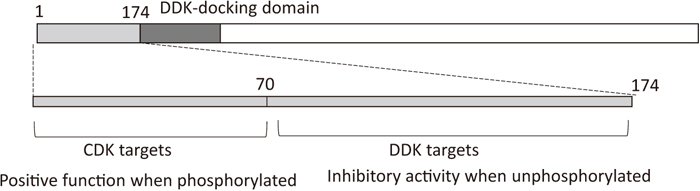
Function of amino-terminal region of Mcm4 from S. cerevisiae. In the amino-terminal region of Mcm4, the upstream region proximal to the DDK-docking domain has a negative role in MCM2-7 function when it is not phosphorylated by DDK (Sheu et al., 2014). Upon phosphorylation, the negative function is neutralized and phosphorylated Mcm4 is recognized by Sld3. CDK phosphorylates serine/threonine in the upstream region distal to the DDK-docking domain to stimulate the function of Mcm2-7.

Protein assembly at the replication origin. At replication origins, DDK phosphorylates Mcm4 and Mcm6 for the interaction with Sld3 bound to Cdc45. CDK phosphorylates Sld2 and Sld3 to assemble a large protein complex through the phospho-acceptor domains of Dpb11.
To activate CMG helicase activity, the assembly and function of MCM10 in the origin region are required. Mcm10 is one of the replication initiation factors and binds to Mcm2 and Mcm6 through its carboxyl-terminal region in S. cerevisiae (Douglas and Diffley, 2016). In higher eukaryotic cells, a direct interaction between MCM10 and MCM2-7 has been demonstrated. We observed that human MCM10 mainly binds to MCM4, 6 and 7 (unpublished results), which may directly bind to single-stranded DNA during DNA unwinding, and we also showed that MCM10 binds to the ATP-binding domain of MCM6 (Hosoi et al., 2016). A recent study showed that MCM10 activity to stimulate DNA replication in human cells requires its two MCM2-7-interacting domains, located in the central domain (amino acids 200 - 482) and carboxyl-terminal region (amino acids 530 - 655) (Izumi et al., 2017). It was also suggested that an interaction of Mcm10 and double-hexameric Mcm2-7 is required for helicase splitting and activation during S phase (Quan et al., 2015). However, results contradictory to this proposal have been reported (Miyazawa-Onami et al., 2017; Douglas et al., 2018).
MCM10 reportedly plays a role in tethering DNA polymerase α at the replication origin and forks. Studies using Xenopus egg extracts indicated that Mcm10 plays an essential role as a scaffold in the progression of the DNA replication fork by tethering replication proteins, and phosphorylation of Mcm10 at S630 with CDK is involved in protein assembly at the fork (Chadha et al., 2016). In elongation during DNA replication, Mcm10 is required for gene silencing and chromosomal condensation (Apger et al., 2010). Mcm10 was shown to bind HP1 (heterochromatin protein 1) through its carboxyl-terminal region.
Over-replication in a single cell cycle is mainly prevented by CDK. CDK phosphorylates Orc2, Mcm2-7 and Cdc6, resulting in their inactivation in S. cerevisiae. It has been suggested that Mcm2-7 is exported from the nucleus to the cytoplasm (Labib et al., 1999), and that Cdc6 is degraded via phosphorylation by CDK. Bypassing these reactions results in over-replication, indicating that CDK redundantly phosphorylates replication proteins to prevent over-replication (Nguyen et al., 2001). It is unknown whether nuclear localization of MCM complexes is regulated during interphase in higher eukaryotes. However, overexpression of Mcm3 can prevent DNA replication in mouse cells through the nuclear exporting signals in the central region of Mcm3, suggesting that the capacity for nuclear export of MCM complexes is present in higher eukaryotic cells (Chuang et al., 2012). It does not appear that phosphorylation of Mcm3 by CDK regulates the nuclear export of Mcm complexes. CDK phosphorylation sites are clustered in the amino-terminal region of human MCM4 (Fig. 7). MCM4 phosphorylation peaks during M phase and is largely counter-correlated with chromatin binding of the MCM complex during the cell cycle (Komamura-Kohno et al., 2006). It also results in inactivation of MCM4/6/7 helicase activity. These results suggest that phosphorylation of MCM4 by CDK has a negative effect on MCM function in higher eukaryotic cells. Overall phosphorylation of MCM4 by CDK may inhibit the binding of MCM2-7 to chromatin during the S and G2/M phases, and the reaction may contribute to ensuring a single round of DNA replication in a cell cycle. Consistent with this proposition, cells harboring mutant MCM4 in which amino-terminal regions (1 - 50 or 1 - 74) are genetically deleted were shown to possess a DNA content greater than 4N, suggesting that over-replication occurs in these cells (Gineau et al., 2012).

Phosphorylation of human MCM4 by CDK. (A) The amino acid sequence of the amino-terminal region of human MCM4 is shown, with phospho-acceptor sites for CDK underlined. (B) Phosphorylation of MCM4 at six sites was analyzed for cell-cycle dependency (Moritani and Ishimi, 2013), reactivity to checkpoint reaction (Ishimi et al., 2004), possible reactivity to DDK phosphorylation after CDK phosphorylation, and relationship to cell growth. S: S phase, M: M phase, S-M: from S to M phase. ND: not determined.
A total of 12 sites for phosphorylation by CDK are present in the amino-terminal region of human MCM4. We analyzed six of these sites (3, 7, 19, 32, 54 and 110). Site 3 was mainly phosphorylated during S phase; sites 7, 32 and 54 were phosphorylated during S and M phases; and those at 19 and 110 were mainly phosphorylated during M phase (Moritani and Ishimi, 2013). Phosphorylation of MCM4 by CDK2 at these six sites is enhanced in a checkpoint-dependent manner when DNA replication is perturbed (Ishimi et al., 2004). Phosphorylation at sites 19 and 110 in chromatin-bound MCM4 was greatly enhanced under these conditions, suggesting that phosphorylation at these two sites plays a negative role in MCM function. Consistent with this finding, phosphorylation of MCM4 at site 19 was rarely detected in rapidly growing human cells, but was detected in aged cells (data not shown). DDK requires acidic amino acids adjacent to Ser or Thr of phospho-acceptor amino acids for phosphorylation. In the amino-terminal region of human MCM4, several (S/T)(S/T)P sites are present (Fig. 7A). When the second S/T site is phosphorylated by CDK, it mimics an acidic residue and the first S/T may then be phosphorylated by DDK. In the case of MCM2, which has three SSPs in the amino-terminal region (Fig. 4A), its phosphorylation by DDK is facilitated by prior phosphorylation of the MCM2/4/6/7 complex by CDK (Masai et al., 2000). Thus, phosphorylation of MCM4 by CDK at sites 3, 7, 32 and 54 may stimulate phosphorylation at sites 2, 6, 31 and 53 by DDK. Therefore, phosphorylation by CDK at 3, 7, 32 and 54 may play a positive role in MCM function. Partly consistent with this notion, phosphorylation at 3 and 32 occurs near sites of DNA synthesis (Komamura-Kohno et al., 2006). In S. cerevisiae, phosphorylation of Mcm4 in the amino-terminal region plays positive roles in DNA replication (Sheu et al., 2014).
At the termination of DNA replication, where two progressive DNA replication forks meet on the genome, CMG helicase function must be inhibited to prevent over-replication. Recently, it has been reported that MCM7 is polyubiquitylated and then evicted through the action of the CDC48 protein complex, which results in detachment of the CMG complex from chromatin (Moreno et al., 2014). E3 ubiquitin ligase was found to be responsible for this reaction (Dewar et al., 2017). It remains unclear how MCM7 in CMG helicase at the termination of DNA replication is selectively recognized by E3 ligase. MCM2-7 function is regulated by various conditions including the time at which DNA replication elongation is inhibited. The proteins responsible for MCM regulation have been identified. We found that these proteins commonly interact with the ATP-binding domain of MCM6 (Fig. 8) (Hosoi et al., 2016). Among them, MCM-BP has been identified as a novel member of MCM2-7 family proteins but does not have canonical sequences for ATP binding. MCM-BP interacts with the amino-terminal half and ATP-binding domain of MCM6. In the absence of MCM-BP, chromatin-bound MCM2-7 accumulates in the DNA replication system in Xenopus egg extract (Nishiyama et al., 2011), suggesting that MCM-BP is involved in the detachment of MCM2-7 from the chromatin. MCM-BP also interacts with MCM7 among MCM2-7 proteins and with SMC3 in HeLa cells (Kusunoki and Ishimi, 2014). This finding supports the notion that MCM-BP interacts with MCM2-7 at replication forks to regulate the function of the complex and thereby stimulates re-formation of the cohesion complex that bundles sister chromatids.
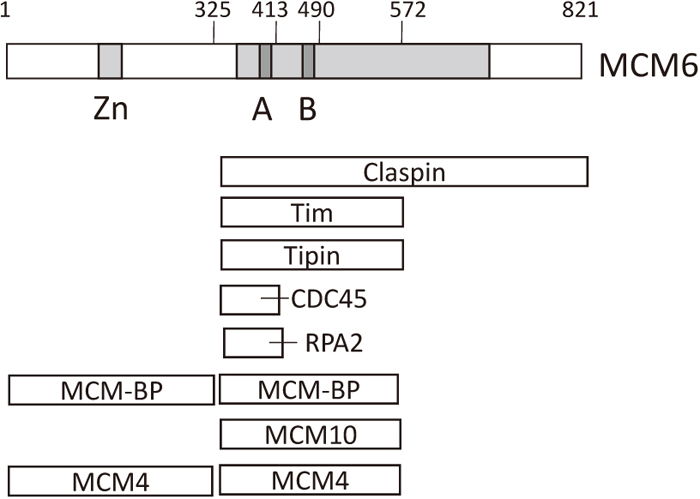
Regions in MCM6 where MCM-interacting proteins bind. Binding of MCM-interacting proteins to several MCM6 fragments has been examined. The smallest MCM6 fragments that bound to each of the interacting proteins are indicated (Hosoi et al., 2016). Claspin, Tim and Tipin form a fork protection complex that inhibits MCM2-7 function, which in turn inhibits progression of replication forks (Leman and Noguchi, 2012). RPA2 is the middle subunit of the heterotrimeric RPA complex that binds to single-stranded DNA at the replication fork. The light gray area containing the MCM box corresponds to the ATP-binding domain.
During the G1 phase of the cell cycle, CDK phosphorylates Rb to activate transcription factor E2F, and MCM2-7 mRNA is synthesized through the action of E2F. In aged cells, MCM2-7 protein levels dramatically decrease (Dumit et al., 2014). Loss of replication activity in aged cells is mainly caused by a DNA damage checkpoint system, which is triggered by shortened telomeres. Down-regulation of DNA replication proteins may also contribute to the loss of replication activity. In aged cells, microRNAs that degrade MCM2-7 mRNA are synthesized via the action of p53 (Bai et al., 2016). Decreased levels of MCM2-7 proteins in vivo have been reported in aging hematopoietic stem cells, and data suggest that this process plays a causative role in cell aging by inducing replication stress (Flach et al., 2014). Cell proliferation is regulated by growth stimuli, and cells are arrested in G0 in the absence of stimuli. Low levels of Mcm2-7 proteins have been detected in the G0 phase in Schizosaccharomyces pombe (Namdar and Kearsey, 2006). Cell growth of human MCF-7 cells derived from a mammary carcinoma is stimulated in the presence of estrogen. Many MCF-7 cells in the absence of estrogen show low levels of MCM2-7 proteins (Tsuji et al., 2018). In the presence of estrogen or estrogen-like molecules, MCM2-7 mRNA expression increased. Under these conditions, signal transduction of the PI3K-AKT-GSKβ-cyclin D1-Rb pathway may be stimulated (Fig. 9).

Signal network from growth stimulation to MCM proteins. PI3K activated by growth stimuli phosphorylates and activates AKT. AKT phosphorylates GSK-3β to inhibit its phosphorylation of cyclin D1. Unphosphorylated cyclin D1, which is nuclear-localized, phosphorylates Rb to release E2F from Rb. E2F functions as a major transcription factor for MCM2-7 genes. In aged cells, activated p53 induces transcription of microRNAs that degrade MCM2-7 mRNA. Under hypoxic conditions, HIF-1 interacts with MCM and CDC6 to inhibit the initiation of DNA replication.
Under hypoxic conditions, cell growth is inhibited and gene expression to adapt to these conditions is induced. The heterodimeric hypoxia-inducible factor (HIF)-1 transcription factor is mainly responsible for this gene regulation. HIF-1α directly interacts with MCM2-7 and CDC6 (Hubbi et al., 2011, 2013). Chromatin-bound MCM2-7 increases through the action of HIF-1, whereas DNA replication is inhibited, under hypoxic conditions, suggesting that HIF-1 inhibits the initiation of DNA replication at a step after assembly of the pre-replication complex. It was suggested that MCM2-7 mRNA expression is negatively regulated by HIF-1 under hypoxic conditions. Under normal conditions, HIF-1α protein is degraded via proline hydroxylation-dependent polyubiquitylation. MCM7 stimulates the polyubiquitylation of HIF-1 by interacting with its central domain, and MCM3 inhibits HIF-1α transcriptional activity by interacting with the region near the carboxyl-terminal transactivation domain (Hubbi et al., 2011). Thus, the interaction between HIF-1 and MCM2-7 plays important roles in regulating cell growth under conditions where the O2 concentration varies. Further analysis will be required for understanding the interaction between HIF-1 and MCM2-7 proteins.
Accumulating evidence indicates that MCM2-7 proteins are among the most reliable diagnostic markers of cancer. In some cancer cells, MCM2-7 proteins are overexpressed (Ishimi et al., 2003; Seo and Kang, 2018), suggesting their important roles in cancer progression. Consistent with this notion, induced overexpression of MCM7 stimulates cell transformation (Honeycutt et al., 2006). MCM7 plays a critical role in the ATPase activity of MCM2-7 helicase (You et al., 2002), and down-regulation of MCM7 but not of MCM2-6 results in growth inhibition (Crevel et al., 2007), indicating that MCM7 is particularly important for MCM2-7 helicase function and cell growth.
Cancer cells are produced by numerous mutations in tumor suppressor genes and proto-oncogenes. In addition, structural abnormalities in the genome play causative roles in cancer cell production. Stalling in the progression of DNA replication forks is a major factor in generating double-stranded DNA breaks, which leads to structural abnormalities in the genome. Shima et al. (2007) reported that a point mutation (F345I) in Mcm4 causes various cancers including mammary carcinoma in mouse. The F345I mutation in Mcm4, located near the Zn-finger motif, weakens the interaction with Mcm6 (Kawabata et al., 2011; Chuang et al., 2012). Analysis of the same mutant MCM4 in human supports that conclusion (Fig. 10) (Watanabe et al., 2012). As a result, both the nuclear-localized MCM2/4/6/7 complex and the MCM2-7 complex bound to origin DNA may decrease, resulting in the generation of unreplicated DNA in S phase and segregation of a structurally altered genome to daughter cells.

Effects of point mutations in human MCM4 on its activity. Effects of point mutations of MCM4 on its binding to MCM6 or MCM7, on DNA helicase activity of the MCM4/6/7 complex and on nuclear localization of MCM4 are shown (Ishimi and Irie, 2015; Tatsumi and Ishimi, 2017). The F346I mutation is the same as the mutation of F345I in mouse Mcm4 that causes various cancers in mouse (Watanabe et al., 2012). The point mutations G364R and G486D of MCM4 are derived from skin cancer cells and cancer cells from the endometrium, respectively.
Systematic analyses of gene mutations in cancer cells have been carried out for numerous cancer-related genes by the Sanger Institute. Mutations were identified in major cancer-related genes and in MCM2-7 genes. We evaluated MCM4 mutations in conserved amino acids and examined the effect of MCM4 point mutations (G364R and G486D) on DNA helicase activity of the MCM4/6/7 complex (Fig. 10) (Ishimi and Irie, 2015; Tatsumi and Ishimi, 2017). The G364R mutation was detected in human skin cancer cells (Ishimi and Irie, 2015) and the G486D mutation was detected in cancer cells from endometrium (Tatsumi and Ishimi, 2017). The G364R mutation did not affect MCM complex assembly but affected DNA helicase activity of the MCM4/6/7 hexamer. The mutation is located in a loop structure that is involved in interaction of adjacent MCM subunits (Brewster et al., 2008). The G486D mutation, located in the conserved MCM box, weakens the interaction with MCM7, preventing formation of the MCM4/6/7 complex. G486D MCM4 forcibly expressed in HeLa cells does not localize to chromatin, and its expression induces the generation of abnormal nuclear structures, suggesting that the mutant MCM4 affects DNA replication by perturbing MCM2-7 function. These dominant-negative effects of mutant MCM4 on HeLa cells have been observed in other MCM4 mutations. These results suggest that these mutations contribute to cancer cell progression by disturbing DNA replication in the cells from which they are derived.
I thank anonymous reviewers for giving constructive comments on this review.