2021 Volume 96 Issue 1 Pages 25-32
2021 Volume 96 Issue 1 Pages 25-32
Small ubiquitin-related modifier (SUMO) is a post-translational modification factor composed of about 100 amino acid residues. Most plant species express a family of SUMO isoforms. We found three novel homologs of rice (Oryza sativa L.) SUMO genes, OsSUMO4, OsSUMO5 and OsSUMO6, in addition to the known SUMO genes OsSUMO1–OsSUMO3. Phylogenetic tree analysis revealed that rice SUMO genes have diverged considerably during their evolution. All six of these SUMO genes complemented the phenotype of the SUMO-deficient pmt3Δ mutant of fission yeast. Among the amino acid sequences of rice SUMO proteins, consensus motifs (ΨKXE/D) of the SUMO acceptor site were found in OsSUMO3, OsSUMO4, OsSUMO5 and OsSUMO6. The heat shock protein HSF7 is known to be SUMOylated in Arabidopsis thaliana. SUMOylation using a bacterial expression system revealed that the rice HSF7 homolog was modified by the six rice SUMOs, and further suggested that OsSUMO1, OsSUMO3, OsSUMO4 and OsSUMO6 are involved in its multiple SUMOylation.
Many kinds of proteins are modified post-translationally by mechanisms such as protein phosphorylation, acetylation, glycosylation, ubiquitylation and SUMOylation (Kerscher et al., 2006; Wang et al., 2014). Small ubiquitin-related modifier (SUMO) is a low-molecular-weight protein consisting of approximately 100 amino acid residues, which shows structural similarity to ubiquitin, although the functions of ubiquitin and SUMO are largely different (Kim et al., 2002). Whereas ubiquitylation of proteins is involved in proteasomal degradation (Saeki, 2017), SUMOylation is proposed to suppress the degradation of, and/or to change the intracellular localization of, the target protein (Mahajan et al., 1998; Gill, 2003).
SUMOylation has been investigated in many species (e.g., Johnson, 2004; Colby et al., 2006). In Saccharomyces cerevisiae, the SUMO precursor is initially cleaved in the C-terminal region by a SUMO-specific protease to expose its di-glycine motif (Nayak and Müller, 2014). The resultant mature SUMO is bound to SUMO-activating E1 enzyme, a heterodimer of SAE1 and SAE2, by an ATP-dependent reaction. It is then passed to an E2 SUMO-conjugating enzyme, SCE1 (Lois and Lima, 2005). Finally, SUMO is bound to the target protein at a lysine residue in the acceptor site, resulting in the formation of an isopeptide linkage (Rosas-Acosta et al., 2005). Most SUMO-modified proteins contain the consensus motif ΨKXE/D as the acceptor site, where Ψ is a hydrophobic residue (Lois et al., 2003; Rosa et al., 2018; Morrell and Sadanandom, 2019). SUMO E3 ligase is thought to recognize the target protein to form a SUMO–SCE1 complex and to promote conjugation of SUMO to its target protein. SUMO E3 ligase facilitates this reaction, but SUMO formation can sometimes occur even in the absence of SUMO E3 ligase in vivo and in vitro (Novatchkova et al., 2004; Lois and Lima, 2005; Miura et al., 2005).
SUMOylation has been proposed to have various functions in plants. In Arabidopsis thaliana, a mutant of AtESD4, which encodes a SUMO-specific protease, causes dwarfing with early flowering (Murtas et al., 2003). A mutant of AtSIZ1, the gene encoding the SUMO E3 ligase, increases heat stress sensitivity (Yoo et al., 2006). SUMOylation of BES1 by AtSIZ1 negatively regulates brassinosteroid signaling in A. thaliana (Zhang et al., 2019). In rice, OsSIZ1 is reportedly involved in the regulation of responses to phosphate and nitrogen, and may act as a regulator of the Pi-dependent response (Wang et al., 2015).
Previous reports have indicated that there are eight SUMO genes in the A. thaliana genome (Hay, 2005), and three in the rice genome (Rosa et al., 2018). Arabidopsis thaliana SUMO proteins are classified into two groups involved in mono-SUMOylation (mono-SUMO) and poly-SUMOylation (poly-SUMO) (Mukhopadhyay et al., 2006; Lima and Reverter, 2009; Dou et al., 2011). Those in the mono-SUMO group contain no SUMO acceptor sites in their amino acid sequences, while those in the poly-SUMO group have one or multiple SUMO acceptor sites (Tozluoğlu et al., 2010). In addition, it has been suggested that the functions of the individual SUMO proteins are distinct (Kurepa et al., 2003; Chosed et al., 2007; Meulmeester et al., 2008). Here, we report novel SUMO genes in the rice genome, and the results of investigations on SUMOylation by these SUMOs using a bacterial expression system.
Oryza sativa L. cv. Nipponbare seedlings were germinated at 30 ℃ in a dark chamber, and grown for three days under continuous light (130 μmol m−2 s−1) in a growth chamber. They were then cultivated in a greenhouse.
Construction of plasmids expressing rice SUMO genesTotal RNA was extracted from rice leaves as described previously (Imamura et al., 2007), and a cDNA pool was prepared from 1 μg of the total RNA using a ReverTra Ace cDNA synthesis kit (Toyobo, Osaka, Japan) and an oligo(dT)20 primer. Plasmids that were used for expression of rice SUMOs in fission yeast were constructed using an expression vector plasmid, pSLF273L (Forsburg and Sherman, 1997; Tanaka et al., 1999). Using the cDNA pool, DNA fragments corresponding to the mature protein region in full-length SUMO cDNAs were amplified by PCR using the following primer sets: 5′-GTCGACAGATCTCCCGATGTCGGCCGCCGGGGAGGA-3′ and 5′-ACATTCCTTTTACCCGGGCCTCCAGTCTGGTGGAGCA-3′ for OsSUMO1 (accession number AK058869), 5′-GTCGACAGATCTCCCGATGTCGTCGCCCGCGGGGGA-3′ and 5′-ACATTCCTTTTACCCGGGCCCCCAGTCTGGTGAAGCA-3′ for OsSUMO2 (AK103092), 5′-GTCGACAGATCTCCCGATGTTCGGCCGGTCTGGCAT-3′ and 5′-ACATTCCTTTTACCCGGGCCACCGATCAGCTCCTCGA-3′ for OsSUMO3 (AK107923), 5′-GTCGACAGATCTCCCGATGTCGTCGACGCCGGCGGC-3′ and 5′-ACATTCCTTTTACCCGGGCCGCCCTGCTGGTCGATCG-3′ for OsSUMO4 (this work), 5′-GTCGACAGATCTCCCGATGTCGACGACCTCGCCGAG-3′ and 5′-ACATTCCTTTTACCCGGGCCGCCGATCATAACCGGGA-3′ for OsSUMO5 (this work), and 5′-GTCGACAGATCTCCCGATGTACGGCTGGTCTGGCAT-3′ and 5′-ACATTCCTTTTACCCGGGCCACCGATCAACTCCTCGA-3′ for OsSUMO6 (this work). The protein-coding regions of OsSUMO1, OsSUMO2, OsSUMO3 and OsSUMO4 were identified from the nucleotide data registered in the Rice Annotation Project database (RAP-DB; https://rapdb.dna.affrc.go.jp), and those of OsSUMO5 and OsSUMO6 were from data in the MSU database. The resulting DNA fragments were inserted into pSLF273L using an In-Fusion HD Cloning Kit (Takara Bio, Kusatsu, Japan), and the modified vectors were designated as pSLF-OsSUMO1–pSLF-OsSUMO6.
Complementation testing of SUMO gene functions using the fission yeast pmt3Δ mutantComplementation testing was performed by a method modified from a previous report (Tanaka et al., 1999). To test for ultraviolet light (UV) sensitivity, the Schizosaccharomyces pombe pmt3Δ mutant (JN630) [h− leu1-32 ura4-D18 pmt3Δ::ura4], and the pmt3Δ mutant containing one of the plasmids pSLF273L, pSLF273L-pmt3, pSLF-OsSUMO1, pSLF-OsSUMO2, pSLF-OsSUMO3, pSLF-OsSUMO4, pSLF-OsSUMO5 or pSLF-OsSUMO6, were cultured for 16 h in liquid EMM medium (Tanaka et al., 1999) at 30 ℃. Around 1,000 cells of each strain were then spread on two EMM plates (Petersen and Russell, 2016). One of these plates was UV-irradiated in a Stratalinker (Stratagene, La Jolla, CA, USA) at a dose of 20 J m−2. Plates with or without UV treatment were cultured at 30 ℃ for 4–6 days, and the number of colonies generated were counted to calculate the colony survival ratio. Viability and UV sensitivity were determined from this survival ratio.
Bacterial expression of SUMO proteins and detection of SUMOylation in Escherichia coli cellsThe procedure of SUMOylation using the E. coli expression system was modified based on that reported previously (Okada et al., 2009). His-tagged mature forms of the rice SUMO genes were prepared from plasmids containing the corresponding genes by PCR amplification using the following primer sets: 5′-TTTGAATTCGATGTCGGCCGCCGGGGAGGA-3′ and 5′-TTTAAGCTTGCCTCCAGTCTGGTGGAGCA-3′ for OsSUMO1, 5′-AAAGAATTCGATGTCGTCGCCCGCGGGGGA-3′ and 5′-AAAAAGCTTGCCCCCAGTCTGGTGAAGCA-3′ for OsSUMO2, 5′-AAAGAATTCGATGTTCGGCCGGTCTGGCAT-3′ and 5′-TTTAAGCTTGCCACCGATCAGCTCCTCGA-3′ for OsSUMO3, 5′-AAAGAATTCGATGTCGTCGACGCCGGCGGC-3′ and 5′-TTTAAGCTTGCCGCCCTGCTGGTCGATCG-3′ for OsSUMO4, 5′-TTTGAATTCGATGTCGACGACCTCGCCGAG-3′ and 5′-AAAAAGCTTGCCGCCGATCATAACCGGGA-3′ for OsSUMO5, and 5′-TTTGAATTCGATGTACGGCTGGTCTGGCAT-3′ and 5′-TTTAAGCTTGCCACCGATCAACTCCTCGA-3′ for OsSUMO6. These fragments were inserted into the region between the EcoRI and HindIII sites of pCDFDuet-AtSUMO1-AtSCE1a (Okada et al., 2009) and replaced the region encoding AtSUMO1 with those encoding the rice SUMOs. The resulting six plasmids were designated as pCDFDuet-OsSUMO1(GG)-AtSCE1a through pCDFDuet-OsSUMO6(GG)-AtSCE1a. The gene for the T7- and His-tagged HSF7 protein was prepared from a DNA fragment encoding full-length OsHSF7 (AK101700), which was amplified from the cDNA pool using the primer set 5′-TGAATTCATGGCTGACCAGACCGCTGCT-3′ and 5′-GCTCGAGTTAGGTAGAGTTTGAACCGTCTT-3′. The resulting fragment was inserted into the region between EcoRI and XhoI sites in pET28a (Merck, Darmstadt, Germany), which was then named pET28a-OsHSF7. The plasmids pET28a-GFP-GST and pCDFDuet-GFP-AtSCE1a were constructed for the T7-tagged green fluorescent protein (GFP) and the His-tagged GFP, respectively, in a similar way.
For SUMOylation, three plasmids containing one of the genes for His-tagged SUMOs and AtSCE1a, one of the genes for target proteins such as T7-tagged HSF7 and T7-tagged GFP, and pACYCDuet-AtSAE1a-AtSAE2 (Okada et al., 2009) were combined for E. coli BL21(DE3) transformation. As a negative control, His-tagged GFP was added to the transformation mixture instead of the His-tagged SUMO. Transformants were cultured in LB medium (1% polypeptone, 0.5% yeast extract, 1% NaCl) at 37 ℃ until the suspension reached an optical density at 600 nm of 0.4. Isopropylthiogalactoside (IPTG) was then added to the medium (0.2 mM final concentration), which was incubated for a further 5 h at 25 ℃ to induce protein expression. Cells were harvested and used for further analysis by SDS-polyacrylamide gel electrophoresis (SDS–PAGE).
Protein blot analysisProtein extraction from the E. coli cells and SDS–PAGE were performed according to an established procedure (Ausubel et al., 1987). Proteins were blotted onto polyvinylidene difluoride membranes (BioTrace PVDF; Pall Corporation, Port Washington, NY, USA), and incubated with a rabbit anti-T7 antibody (Sigma-Aldrich, St. Louis, MO, USA) or anti-His-tag monoclonal antibody 9C11 (Wako Pure Chemicals, Tokyo, Japan). Immunoblotted proteins were detected and visualized using the ECL Plus Western Blotting Analysis System (GE Healthcare, Chicago, IL, USA).
Reverse transcription PCR (RT–PCR)RT–PCR was performed using KOD-dash DNA polymerase (Toyobo) and an ABI 2720 thermal cycler (Life Technologies, Carlsbad, CA, USA). Transcripts of the genes for rice SUMOs and Actin1 (AK100267) were amplified by pairs of gene-specific primers: 5′-GGGTCGTCGTCGTCTCCTTCCT-3′ and 5′-TGCCAAGCACCAGCAACGAT-3′ for OsSUMO1, 5′-GCCGACTTCTCCTCCCCCAACC-3′ and 5′-TTAACACCAAAGTACCAA-3′ for OsSUMO2, 5′-CAGCAACACAACTACGCAAGTACA-3′ and 5′-CGGATGGGCTCCTGCTC-3′ for OsSUMO3, 5′-GACGTCTACTTCGCCATCAAGC-3′ and 5′-CGTCGATGAACTTGCGGTCT-3′ for OsSUMO4, 5′-GCTGCAGGTGGTCATGGAC-3′ and 5′-TGCATTGGAAAAATCCTCCG-3′ for OsSUMO5, 5′-GTGAAGGTGGAGAAGGAGAACG-3′ and 5′-CCCGGGCTACGCGAGGA-TCGACGA-3′ for OsSUMO6, and 5′-AGCTTCCTGATGGACAGGTT-3′ and 5′-CACAAGTGAGAACCACAGGT-3′ for Actin1.
Phylogenetic analysisAlignment of protein sequences was performed using Clustal X software (http://www.clustal.org/). The phylogenetic tree was constructed using the PHYLIP version 3.69 neighbor-joining method (http://evolution.genetics.washington.edu/phylip.html) with bootstrap values from 1,000 neighbor-joining bootstrap replicates. The tree was visualized using the TreeView program (Page, 1996).
Three SUMO family genes have been identified in the rice genome: OsSUMO1 (Os01g0918300), OsSUMO2 (Os01g0918200) and OsSUMO3 (Os07g0574500) (Rosa et al., 2018; Morrell and Sadanadom, 2019). We sought other homologs of SUMO genes in the rice genome. BLAST analysis using tblastn (https://blast.ncbi.nlm.nih.gov) suggested that there were three further genes encoding proteins showing similarities in their amino acid sequence to OsSUMO1 (Fig. 1A). These three genes were designated OsSUMO4, OsSUMO5 and OsSUMO6. The amino acid sequences of OsSUMO2, OsSUMO3, OsSUMO4, OsSUMO5 and OsSUMO6 showed 90.5%, 43.0%, 37.2%, 37.5% and 40.7% identity, respectively, to that of OsSUMO1 (Supplementary Table S1). In the amino acid sequences of OsSUMO1–6, consensus motifs (ΨKXE/D) of the SUMO acceptor site were found in OsSUMO3, OsSUMO4, OsSUMO5 and OsSUMO6 (Fig. 1A).

Comparison of the rice SUMOs. (A) Alignment of amino acid sequences of the unprocessed forms of rice SUMOs and S. pombe Pmt3 (Pmt3). Amino acid residues identical to those of OsSUMO1 are shaded. Di-glycine motifs at the end of the mature SUMO proteins are indicated by a white letter G on a black background. Potential SUMOylation sites (ΨKXE/D) are boxed. (B) Detection of transcripts of the six rice SUMO genes. Each transcript was amplified by RT-PCR. 1: young leaves; 2: roots in young plants; 3 and 4: mature leaves; 5: flag leaves; 6: spikelets; 7 and 8: developing seeds (3 and 10 days after flowering, respectively). As a control, the transcript for the rice Actin1 gene was analyzed (bottom panel).
OsSUMO4 has been registered in RAP-DB as Os01g0852300. OsSUMO5 and OsSUMO6 are located close to each other in the genome. OsSUMO5 and OsSUMO6 are shown as two different proteins derived from LOC_Os07g38650 and LOC_Os07g38660 in the MSU Rice Genome Annotation Project. However, OsSUMO5 and OsSUMO6 were registered as a single gene in chromosome 7, Os07g0574200, in RAP-DB. The coding regions for OsSUMO5 and OsSUMO6 were found in tandem in this gene, and separated by a predicted intron (Supplementary Fig. S1). Therefore, it was unclear whether OsSUMO5 and OsSUMO6 comprised one or two polypeptides. Expression profiles of OsSUMO4, OsSUMO5 and OsSUMO6 have not been registered in the RiceXPro database (https://ricexpro.dna.affrc.go.jp). We analyzed the transcripts of these genes in planta using RT–PCR, and those for OsSUMO1, OsSUMO2, OsSUMO3 and OsSUMO5 were found in all tissues, suggesting that they are expressed constitutively (Fig. 1B). OsSUMO4 transcripts were detected in tissues other than spikelets and developing seeds, and OsSUMO6 transcripts in tissues other than developing roots and seeds (Fig. 1B). These two SUMO genes seem to be expressed with some tissue specificity.
Phylogenetic tree analysis using amino acid sequences of plant SUMOs indicated that they were divided into two major groups. One group contained OsSUMO1 and 2 within a broad spectrum of plant SUMOs including AtSUMO1 and 2 (Fig. 2). The other group comprised three subgroups, two of which mainly consisted of SUMOs of Gramineae species; one included OsSUMO3, 5 and 6 and the other OsSUMO4 (Fig. 2).

Phylogenetic tree analysis of SUMO proteins. Amino acid sequences of the unprocessed forms of SUMO proteins were used for this analysis. Names of rice and Arabidopsis thaliana SUMOs are indicated, with prefixes Os and At, respectively, corresponding to the plant species, next to their accession numbers. SUMOs of other species are indicated by the name of the species with their accession numbers. The S. pombe Pmt3 (SpPmt3 BAA32595.1) sequence is included as an outgroup. Numbers at the nodes represent bootstrap values. The scale bar represents the number of substitutions per site.
The S. pombe pmt3Δ mutant, which lacks the pmt3 gene encoding SUMO, is very sensitive to UV stress (Tanaka et al., 1999). As described above, OsSUMO4, OsSUMO5 and OsSUMO6 showed structural similarity to the known SUMOs. To determine whether they could restore the deficiency caused by this SUMO mutation, complement testing was performed using this mutant in which each of OsSUMO1, OsSUMO2, OsSUMO3, OsSUMO4, OsSUMO5 and OsSUMO6 was expressed episomally under the inducible nmt* (nmt41) promoter (Tanaka et al., 1999). We determined the survival ratio of these yeast transformants following UV irradiation. In this test, pmt3Δ cells containing empty pSLF273L vector showed a low survival ratio (22.9%), but this was restored in the transformant yeast containing the wild-type pmt3 gene (61.2%). The survival ratios of yeast transformants containing OsSUMO1, OsSUMO2, OsSUMO3, OsSUMO4, OsSUMO5 and OsSUMO6 were 70.0%, 48.9%, 63.3%, 72.3%, 51.8% and 64.6%, respectively (Fig. 3), similar to the transformant containing pmt3. Thus, these SUMOs substantially complemented the S. pombe pmt3 mutation.
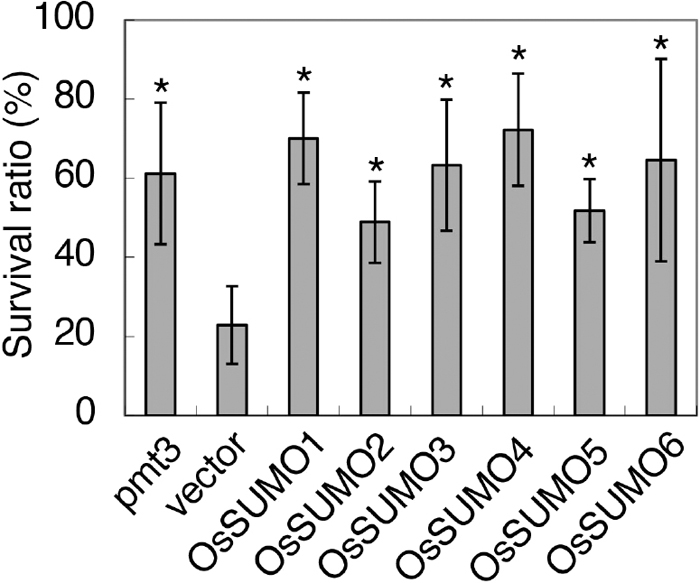
Complementation analysis of rice SUMOs using a fission yeast mutant lacking pmt3. Schizosaccharomyces pombepmt3Δ mutants into which the genes for the mature forms of each of the six rice SUMO proteins had been introduced were irradiated with UV and their survival ability was examined. The survival ratio is shown as the proportion of the number of colonies arising from surviving cells in the total number of treated cells. As positive and negative controls, pmt3Δ cells expressing plasmid-borne wild-type pmt3 (pmt3) and pmt3Δ cells containing empty plasmid (vector), respectively, were used. Error bars represent the mean ± standard deviation (n = 5). Means that differed significantly from the negative control are indicated by asterisks (P < 0.01).
SUMOylation using the E. coli expression system can be established using genes involved in the A. thaliana SUMO pathway (Okada et al., 2009). We analyzed SUMOylation in this system for rice SUMOs in a similar way. The SUMO modification system requires the E1 SUMO-activating enzyme (a heterodimer of SAE1 and SAE2) and the E2 SUMO-conjugating enzyme (SCE1) to conjugate SUMO with its target protein (Novatchkova et al., 2004). The previously reported procedure contains A. thaliana SAE1, SAE2 and SCE1 (Okada et al., 2009). Because these proteins show high similarity to their counterparts in rice, we used the genes AtSAE1a, AtSAE2 and AtSCE1a for our experiments. SUMO modification of the Arabidopsis heat shock protein HSF7 has been reported (Bohren et al., 2004). Because a rice homolog of this protein is presumed to be SUMOylated, we constructed the gene for T7-tagged rice HSF7 and used this protein as a target protein for SUMOylation.
When the SUMOylation reaction was carried out using rice SUMOs, several proteins that were larger than the T7-tagged HSF7 (60 kDa) were detected using protein blot analysis with an antibody against the T7 tag, while no changes in any proteins could be detected in control reactions using T7-tagged GFP instead of T7-tagged HSF7 (Fig. 4). OsSUMO1 and OsSUMO2 have been reported to function as rice SUMOs (Rosa et al., 2018). These results indicated that rice HSF7 is indeed a target of SUMOylation and that our SUMOylation system worked efficiently. In addition to these SUMOs, we detected modification of rice HSF7 in reaction cocktails containing OsSUMO3, OsSUMO4, OsSUMO5 and OsSUMO6, indicating that they were also involved in its SUMOylation. We also detected proteins with molecular sizes larger than that of the mono-SUMOylated HSF7 when OsSUMO1, OsSUMO3, OsSUMO4 and OsSUMO6 were used for the reactions, suggesting that multiple SUMOylation events (poly-SUMOylation) had occurred (Fig. 4A). When the proteins were detected by the antibody against the His tag, proteins with molecular sizes larger than HSF7 were also detected, confirming that SUMOylation had been carried out by the rice SUMOs. Poly-SUMOylation was apparent when OsSUMO3, OsSUMO4 and OsSUMO6 were used for the reactions (Fig. 4B).

SUMOylation analysis using the E. coli expression system. SUMOylation in E. coli cells was investigated using rice HSF7 (T7- and His-tagged) with each of the rice SUMOs (His-tagged). Lanes 1–6 correspond to reactions containing OsSUMO1 to OsSUMO6. The CT lanes contain His-tagged GFP instead of His-tagged SUMO in the reaction. As a negative control, T7-tagged GFP was used instead of T7-tagged HSF7 (GFP lanes). Each panel depicts a protein blot analysis of the reactions, in which tagged proteins were detected using an anti-T7-tag antibody (A) and an anti-His-tag antibody (B). Positions of the T7-tagged HSF7, T7-tagged GFP, His-tagged GFP and possible SUMO-modified T7-tagged HSF7 are indicated as HSF7, GFP(T7), GFP(His) and SUMO-HSF7 on the figure. Sizes (in kDa) of marker proteins (M lanes) are indicated to the left of each blot.
The presence in the rice genome of three SUMOs, OsSUMO1, OsSUMO2 and OsSUMO3, has been reported (Rosa et al., 2018). OsSUMO1 and OsSUMO2 are involved with the salt-stress response (Srivastava et al., 2016), although no functional analysis has been carried out on OsSUMO3. Here, we found three new rice SUMOs and named them OsSUMO4, OsSUMO5 and OsSUMO6 (Fig. 1). SUMOylation using the E. coli expression system indicated that they are involved in protein SUMOylation (Fig. 4). These SUMOs restored resistance to UV stress in the fission yeast pmt3Δ mutant (Fig. 3). Considering the specificity of the SUMOs of different species, their substantial complementation of the pmt3Δ mutation suggested that these SUMOs are functional in vivo.
Generally, multiple SUMOs are found in many plant species. In A. thaliana, eight SUMO genes have been reported, although no transcripts of four of these have been found (Kurepa et al., 2003). Our findings confirm that rice also has multiple SUMOs. The functions of individual SUMOs have diverged widely (Hay, 2005). Thus, A. thaliana AtSUMO3 cannot complement the function of AtSUMO1 or AtSUMO2 (Kurepa et al., 2003). The rice SUMOs were classified evolutionarily into different clades (Fig. 2), and this may imply their functional diversity. OsSUMO3, 5 and 6 and OsSUMO4 were classified into two groups mainly composed of SUMOs of Gramineae species (Fig. 2), suggesting that they arose after the monocotyledons had evolved. This might also signify that the functions of these SUMOs are important for the Gramineae.
Some of the maize SUMOs are involved in poly-SUMOylation, by which SUMO proteins are multiply conjugated to the target protein to form a polymeric SUMO chain (Augustine et al., 2016). In mammalian cells, the activities of SENP6 and SENP7 are enhanced by such poly-SUMOylation (Mukhopadhyay et al., 2006; Lima and Reverter, 2009). There is a SUMO acceptor site in OsSUMO3, OsSUMO4, OsSUMO5 and OsSUMO6, whereas none was found in OsSUMO1 or OsSUMO2 (Fig. 1).
SUMOylation using the E. coli expression system has been established for the A. thaliana SUMOs (Okada et al., 2009). Here, we attempted to apply this methodology to the rice SUMOs. This system contained the A. thaliana E1 and E2 enzymes. They also worked for rice SUMOs involved in protein modification (Fig. 4). This indicates that they might commonly facilitate SUMOylation using SUMO molecules. We observed modification of rice HSF7, from which proteins with larger molecular sizes were generated (Fig. 4).
HSF7 has two potential SUMOylation sites (Supplementary Fig. S2), and is expected to be modified at these sites. We detected a ladder of proteins showing larger molecular sizes than that of HSF7, suggesting that SUMOylation occurs at these sites on HSF7 (Fig. 4). When the reactions were performed in the presence of OsSUMO1, OsSUMO3, OsSUMO4 and OsSUMO6, ladders of multiple proteins were also generated, corresponding to HSF7 with the addition of multiple SUMO molecules. Reactions using OsSUMO3, OsSUMO4 and OsSUMO6 yielded larger proteins corresponding to the HSF7 protein attached to more than three SUMO molecules (Fig. 4). This result indicates that these three rice SUMOs are involved in poly-SUMOylation and/or SUMOylation at multiple SUMO acceptor sites.
We thank C. Mitsuhashi, T. Imamura, K. C. She, T. Sasaki, K. Matsumoto and members of Dr. Tanaka’s laboratory for technical assistance.