INTRODUCTION
Many cultivars of radish, which is one of the most important vegetables, have been established by several seed companies in Japan, and are cultivated all over the world. The shape of the root is highly diverse among these cultivars (Mitsui et al., 2015). The root contains various secondary metabolites, glucosinolates (which are precursors of chemical defense compounds), biopesticides and flavor compounds (Halkier and Gershenzon, 2006). In addition to the agricultural importance of radish, naturalized radish populations are observed in coastal areas in Japan, and their genetic diversity in allozymes and cytoplasmic male sterility-related genes has been partially characterized (Huh and Ohnishi, 2001; Yamagishi and Terachi, 2001). Wild populations of radish are also found in the USA, Canada and Australia, and the diversity in their S alleles, which regulate self-incompatibility traits, as described below, has been characterized (Sampson, 1967; Karron et al., 1990).
Self-incompatibility is an important trait to maintain genetic diversity within species (Watanabe et al., 2012). The self-incompatibility trait in cruciferous plants, including radish, cabbage and turnip, is controlled by a single locus with multiple S alleles (Bateman, 1955). To date, genes encoding the male and female S determinants have been identified and characterized as encoding a small cysteine-rich protein (SP11/SCR) and a receptor-like protein kinase (SRK), respectively (Stein et al., 1991; Watanabe et al., 1994; Schopfer et al., 1999; Suzuki et al., 1999; Takasaki et al., 2000; Takayama et al., 2000, 2001; Shiba et al., 2001). At the S locus, in addition to the two genes encoding SP11 and SRK, at least two additional genes, SLG and Smi1/2, are also located. SLG is required for full manifestation of self-incompatibility reactions (Takasaki et al., 2000), and Smi1/2 encodes a small RNA that regulates the dominance relationship between S alleles on the pollen side (Hatakeyama et al., 1998b; Kakizaki et al., 2003; Tarutani et al., 2010; Yasuda et al., 2016). The S alleles SP11, SRK and SLG are divided into two classes (class-I and class-II; Nasrallah and Nasrallah, 1993), and the sequence identity at the nucleotide level within each class is higher than that between the classes (Hatakeyama et al., 1998a; Watanabe et al., 2012). Because of the complex nature of the S locus, as described above, the S alleles are referred to as the S haplotypes (Nasrallah and Nasrallah, 1993). In addition to the S locus, the Brassica genome contains several SRK-like genes, which are termed the S multigene family (Suzuki et al., 1995, 1997). BcRK6, one of the SRK-like genes, encodes a female determinant of unilateral incompatibility in B. rapa (Kai et al., 2001; Takada et al., 2017).
In tandem with the molecular dissection of the S locus, analysis of genetic diversity and distribution of S alleles in several cruciferous plants (B. rapa, B. oleracea, Sinapis arvensis, etc.) was performed by diallel crosses and test crosses (Bateman, 1955; Thompson, 1957; Sampson, 1967; Ockendon, 1974, 1980, 1982; Mackay, 1977; Wallace, 1979; Visser et al., 1982; Stevens and Kay, 1989; Nou et al., 1991, 1993). In radishes from both wild populations and cultivars, genetic diversity and distribution of S alleles have also been characterized (Sampson, 1957, 1964, 1967; Karron et al., 1990; Niikura and Matsuura, 1998). After identification and characterization of S locus genes, the S genotype was determined from the nucleotide sequence polymorphism of SLG or SRK in Brassica and Raphanus species (Brace et al., 1994; Sakamoto et al., 1998; Park et al., 2002; Kim and Kim, 2019). Recently, two research groups independently defined the S alleles (S haplotypes) in radish according to the S locus genes SLG, SRK and SP11 (Haseyama et al., 2018; Kim and Kim, 2019). Based on their data, at least 31 different S alleles have now been identified and characterized in radish cultivars. Although the distribution and frequency of S alleles have been surveyed in many cultivars and naturalized populations of radish using pollination tests or PCR methods, there are no published data on the distribution and frequency of S alleles in relation to the in situ positions of the plants in naturalized populations.
In this study, we dissected the distribution of S alleles of naturalized radish (R. sativus L. var. raphanistroides) populations in Yakushima, a small island in the East China Sea at latitude 30° 28′ N and longitude 130° 30′ E. In October 2019 and March 2020, seeds and/or leaves were sampled from the naturalized populations and the sampled positions were recorded. Their S alleles were determined by comparison with the SRK partial sequences amplified by PCR. We discuss the characteristics of the distribution of S alleles combined with the positions of the plants in the naturalized populations of Yakushima.
MATERIALS AND METHODS
Plant materials, sampling and conditions
The tester lines for S alleles used in this study included three lines (Pekinmizu (S204 × unidentified S allele), No. 5 (S205 × S209) and Eisai ((S202 × S206) × (S201 × S203))) from Tohoku Seed Co. (Niikura and Matsuura, 1998), and four lines (Kanpaku (S1, S4), Shinhasshu (S1, unidentified S allele), YR Kurama (S2, S3, S4) and Oshin (S10, S15, unidentified S alleles)) from Takii & Co. (Sakamoto et al., 1998) (Supplementary Table S3). These plants were grown in an artificial growth chamber under 16-h light and 8-h dark conditions at Tohoku University, Sendai. Leaves were sampled and used for genomic DNA extraction. Radish seeds and plants growing in Yakushima were sampled in 2019 and 2020. In October 2019, siliques of radish plants were sampled in the coastal areas. The sampled seeds were germinated on petri dishes in December 2019. The germinated seeds were transferred to plastic pots and were grown in an artificial growth chamber under 16-h light and 8-h dark conditions until flowering; 80 (23 from Haruta and 57 from Tashiro) of these plants were used in the experiments. In March 2020, leaves were sampled from 54 flowering plants (12 in Tsukazaki, 12 in Kurio, six in Nakama, five in Haruta and 19 in Tashiro), and a plant with green siliques was sampled as a representative from each area.
Observation of pollen tube behavior
To observe pollen tube behavior, the aniline blue staining method described by Wang et al. (1994) was used. Briefly, after pollination, flowers were mounted on a 1% agar plate. Softened pistils incubated in 1 M NaOH were stained with basic aniline blue solution. The pistils were mounted on a glass slide and observed by fluorescence microscopy (Axio Imager A2, Zeiss, Germany).
Detection of S alleles by SRK partial sequencing
Genomic DNA from leaves or seeds was extracted according to Watanabe et al. (1992) and Takada et al. (2005) with slight modification. Extracted DNA was used as a template for PCR amplification. PCR amplifications of SRK partial sequences were performed using four different sets of SRK-specific primers, designed based on exon 4 and exon 7 sequences (Supplementary Table S2). The sequences of two sets of primers were from Kim and Kim (2019), and the other sets were designed based on a multiple sequence alignment of several SRK nucleotide sequences of B. rapa (Supplementary Table S2). PCR amplifications were performed with Ex Taq DNA polymerase (TaKaRa, Japan) with pre-denaturation for 4 min at 95 ℃, followed by 35 cycles of denaturation for 30 s at 95 ℃, annealing for 30 s at 57 ℃ and extension for 60 s at 72 ℃, with a final extension at 72 ℃ for 10 min, using a TP650 DNA thermal cycler (TaKaRa) according to Kim and Kim (2019) with slight modification. The nucleotide sequences of the PCR products were determined by direct sequencing using a DNA sequencer (Genetic Analyzer 3500; ABI Hitachi, Japan). Products containing mixed sequences amplified from multiple S alleles were cloned into plasmid vectors (pTAKN2, BioDynamics Laboratory, Japan). The obtained data were analyzed by BLAST (https://www.ncbi.nlm.nih.gov/; Altschul et al., 1997; Johnson et al., 2008). In cases where the highest sequence identity against a known SRK sequence of R. sativus exceeded 99%, the determined SRK sequence was assigned to a known SRK allele based on Kim and Kim (2019) (Supplementary Table S1). On the other hand, in cases where the highest identity against any known SRK sequence was below 98%, we classified the SRK sequence as a novel allele that has not been identified previously (Supplementary Table S1). The partial nucleotide sequences determined in this study for RsSRK34, RsSRK35, RsSRK36 and RsSRK37 will be available in the DDBJ/EMBL/GenBank databases with the accession numbers LC591983, LC591984, LC591985 and LC591986, respectively.
Construction of a phylogenetic tree
Multiple sequence alignment of SRK sequences of Brassica and Raphanus species was performed using MAFFT (Katoh et al., 2019). Suspicious regions of aligned sequences were removed using trimAl (Capella-Gutiérrez et al., 2009). A phylogenetic tree was constructed using the neighbor-joining method (Saitou and Nei, 1987). To evaluate clustering patterns, a bootstrap probability was calculated with 1,000 resamplings. The phylogenetic tree was visualized using iTOL (Letunic and Bork, 2019).
RESULTS AND DISCUSSION
Confirmation of the method of PCR amplification of SRK partial sequences in seven radish cultivars
Before analyzing the S alleles of radish plants sampled in Yakushima, the utility of the PCR primer sets described in Materials and Methods was confirmed. We selected SRK for genotyping of S alleles because the nomenclature of S alleles and sequences of the kinase domain of SRK in radish are well established (Kim and Kim, 2019) (Supplementary Table S1). We did not attempt genotyping of SP11 because the nucleotide sequences are highly diverse among the alleles, making the design of useful primer pairs difficult (Suzuki et al., 1999; Takayama et al., 2000; Watanabe et al., 2000). Genomic DNA extracted from seven radish cultivars established by two Japanese seed companies, Tohoku Seed Co. and Takii & Co., was used as a template for PCR amplifications of SRK partial sequences. Nomenclature of S alleles in radish cultivars was established by Niikura and Matsuura (1998) and Sakamoto et al. (1998). For PCR amplification of SRK partial sequences, two primer sets, PK and KK (Supplementary Table S2), were used. The nucleotide sequences of the SRK alleles amplified from each cultivar using either the PK or KK primer set (Supplementary Table S3) were identical, indicating that in addition to the KK primer set (Kim and Kim, 2019), the PK primer set is applicable for analysis of radish SRK sequences. Thus, in this study, we analyzed S alleles in radish from Yakushima using both PK and KK primer sets.
Interestingly, in five cultivars (Eisai, Kanpaku, Shinhasshu, YR Kurama and Oshin) an SRK-like sequence (tentatively named SRK-#38), registered as an SRK allele of Raphanus raphanistrum in the DDBJ/EMBL/GenBank databases (accession no. KP117077), was amplified with the class-II primer sets (data not shown). PCR amplification of an SRK-like sequence was also reported by Kim and Kim (2018) and Kim and Kim (2019). Kim and Kim (2019) reported that RsSRK-like8, which was identified from a radish draft genome as an SRK homolog by Kim et al. (2016), was amplified by the class-II primer set as a non-S locus gene. Moreover, it was reported that RsKD1, which was identified from another radish draft genome as a gene showing high sequence identity in the kinase domain of the SRK, might complicate S genotyping because RsKD1 is a non-S locus gene and could be amplified with class-II SRK primers (Kim and Kim, 2018). Our SRK-like sequence (SRK-#38) displayed over 99% identity with RsSRK-like8 and RsKD1 in BLAST analysis using the nucleotide sequences. Therefore, SRK-#38 is not an SRK allele and was eliminated as an SRK allele in this study. SRK-#38 may belong to the S multigene family (Suzuki et al., 1995, 1997), with an unknown function in radish.
In two of the cultivars, Pekinmizu and No. 5, two SRK alleles were amplified (Supplementary Table S3). The combination of S alleles was class-I and class-II S heterozygote in both these cultivars. The SRK alleles of Kanpaku and Shinhasshu were identical (Supplementary Table S3). The cultivar Eisai was established by a double-way crossing system, meaning that two different S heterozygotes, (S202 × S206) × (S201 × S203), were crossed in hybrid seed production. However, only two different S alleles, RsS2 and RsS10, were detected in Eisai (Supplementary Table S3). This may have been due to the findings in previous studies that some SRK alleles in radish are difficult to detect because of nucleotide sequence diversity in the S locus genes (Okamoto et al., 2004; Kim et al., 2016; Haseyama et al., 2018).
Thus, the method of PCR amplification of SRK partial sequences could be applied to our radish samples based on the analysis and assignment of S alleles using the SRK partial sequences classified by Kim and Kim (2019), although there were several SRK alleles that could not be amplified and characterized, as also described below.
Characterization of two naturalized radish populations in October 2019 in Yakushima
In October 2019, to sample siliques of radish, we surveyed seven areas in Yakushima (Tsukazaki (Tk), Kurio (Kr), Nakama (Nk), Hara (Hr), Haruta (Hh), Tashiro (Ts) and Kusukawa (Ks); Fig. 1). In only two areas, Haruta and Tashiro, could fallen siliques be sampled in the coastal areas (Fig. 1).

From the seeds sampled in Haruta and Tashiro, 23 and 57 plants, respectively, were grown in pots for use in experiments. To observe pollen tube behavior in self- and cross-pollination, aniline blue staining was performed. Nine plants were selected randomly and used for self-pollination tests. All plants showed the incompatible phenotype, i.e., inhibition of pollen tube penetration on papilla cells, as exemplified by plant #20-12-2 (Fig. 2A), suggesting that both the male and female S determinants were functional. Moreover, most of the plants of the Haruta and Tashiro populations are expected to be self-incompatible, because if these populations contained any self-compatible plants, the self-compatibility trait would spread rapidly within the populations (Igic et al., 2008). In the case of the cross #10-2-1 (female) and #5-1-1 (male), the compatible phenotype, i.e., pollen tube penetration and elongation, was observed (Fig. 2B). To confirm this compatible result, we determined the S genotype of the two plants: #10-2-1 had RsS14/RsS17 and #5-1-1 had RsS8/RsS25. RsS14 and RsS25 are classified as class-I, and RsS8 and RsS17 are classified as class-II (RsS8, RsS14 and RsS25 were found in Tashiro in 2020, but RsS17 was not, as described below). Because class-I alleles are dominant over class-II alleles on the pollen side, the pollen phenotype would be RsS25 and was thus different from the stigma phenotype, RsS14/RsS17. Therefore, this pollination would be compatible, as observed in the pollination test (Fig. 2B).

In the analysis of the plants sampled in October 2019, our aim was to determine the potential for amplification with the class-I and class-II primers in the populations. In the PCR amplification of SRK, three patterns were possible: amplification with only the class-I primer set, with both class-I and class-II primer sets (class-I/class-II), and with only the class-II primer set. Each plant in the samples from Haruta and Tashiro was classified according to the PCR amplification patterns, and the number of plants in each pattern was recorded (Fig. 3). The distribution of the PCR amplification patterns was different between Haruta and Tashiro. In Haruta, two patterns, class-I/class-II and class-II only, were present equally. In Tashiro, however, the class-I/class-II pattern was present at a higher frequency. To determine the independence of the distribution pattern between the two populations, Pearson’s chi-squared test was performed. The test value (chi-squared = 9.79, P < 0.05, df = 2) confirmed that the distribution pattern was different, suggesting that each population had a specific allelic distribution.

We observed plants with flowers and developing siliques in seven areas (Tsukazaki, Kurio, Nakama, Hara, Haruta, Tashiro and Kusukawa) in March 2020 (Supplementary Fig. S1A and S1B). To dissect the S allele variation in depth, we sampled plants in five of these areas (Tsukazaki, Kurio, Nakama, Haruta and Tashiro); these areas were selected because Haruta and Tashiro are located on the east side of Yakushima, whereas Tsukazaki, Kurio and Nakama are on the west side (Fig. 1). The number and positions of plants in randomly placed 1 m × 1 m quadrats were recorded. The S alleles of each plant sampled in the five areas were determined according to SRK partial sequences, as described in Materials and Methods (Fig. 4 and 5A, Supplementary Fig. S2, Supplementary Table S4).

Combining data from the five areas, six additional SRK alleles not described by Kim and Kim (2019) were detected; these S alleles were named RsSRK32 to RsSRK37 (to follow in sequence after the previously identified RsS31; Supplementary Table S1). Among the six alleles, RsSRK32 is registered as an SRK of Raphanus sativus in the databases and RsSRK33 was reported by Haseyama et al. (2018) (Supplementary Table S1). Interestingly, four S alleles (RsS34 to RsS37) were novel, and may therefore be unique to Yakushima. Alignment and comparison with deduced amino acid sequences of SRK deposited in the databases suggested that RsSRK34 to RsSRK37 are functional genes (Supplementary Fig. S3).
On the west side of Yakushima, Kurio is located between Tsukazaki and Nakama (Fig. 1). In Kurio, seven S alleles were found and five of them (71%; RsS7, RsS12, RsS14, RsS22, RsS33) were shared with Tsukazaki and/or Nakama, suggesting that the three areas are genetically related (Fig. 4, Table 1). On the east side of Yakushima, Haruta is located next to Tashiro (Fig. 1). In Haruta, four S alleles were found and two of them (50%; RsS13, RsS23) were shared with Tashiro, suggesting that the two areas on the east side of the island are genetically related, as was found on the west side (Fig. 4, Table 1). Eleven S alleles were found in the west side whereas 12 S alleles were found in the east side (Fig. 4, Table 1). From a comparison of S alleles between the west and east sides, three S alleles (27% in the west side, 25% in the east side; RsS8, RsS14, RsS28) were found to be shared. These data indicate that the number of shared S alleles was correlated with the distance between populations. During the research in Yakushima, it was observed that western honeybees (Apis mellifera), a potential pollinator, foraged flowers of these plants (data not shown). Their average foraging distance has been estimated to be about 1.5 km (Steffan-Dewenter and Kuhn, 2003), which is considered to be a potential pollination range. Also, our data suggest that the diversity of S alleles is maintained locally within areas in Yakushima.
Table 1. Number of
S alleles identified in samples from five different areas in March 2020
| Area | Number of identified S alleles |
|---|
| Tsukazaki | 7 |
| Kurio | 7 |
| Nakama | 4 |
| Haruta | 4 |
| Tashiro | 10 |
| Total | 20 |
Most of the S alleles detected in the five areas were classified as class-I (Supplementary Table S4). However, the number of identified S alleles varied in the five areas (Table 1). If the number of plants in a population decreases, the number of S alleles that are lost in the population would be expected to increase (Byers and Meagher, 1992). The number of S alleles identified in the higher population density areas, Tsukazaki, Kurio and Tashiro, was indeed larger than the number in the lower population density areas, Haruta and Nakama (Fig. 5A, Table 1, Supplementary Fig. S2). Therefore, our data support the previous study by Byers and Meagher (1992).
When we compared the identified S alleles in the cultivars and the naturalized plants, only five, RsS2, RsS7, RsS8, RsS12 and RsS17, were common to both (Fig. 4, Supplementary Tables S3 and S4). However, RsS1 to RsS31 have previously been identified in cultivars, as described by Kim and Kim (2019) (Supplementary Table S1). Thus, most of the S alleles were common to cultivars and the naturalized populations. Interestingly, plants that are intermediate between radish cultivars and radish plants in naturalized populations were also observed in Yakushima and other locations (data not shown). These observations suggest that exchange of S alleles between cultivars and naturalized plants has occurred.
The SRK-like sequence SRK-#38, which was the same as in the cultivars, was amplified from all plants sampled in March 2020 (data not shown). Moreover, in only 12 plants (Tk9, Kr1, Kr2, Kr3, Kr6, Kr7, Kr8, Kr12, Nk1, Nk2, Ts2 and Ts4), two SRK alleles were amplified in addition to SRK-#38 (Supplementary Table S4). These results confirmed that SRK-#38 is not an allele of the S locus, as discussed above. The low number of plants in which two SRK alleles were amplified (12 of 54 plants) indicates that there were undetectable SRK alleles in most of the plants of the naturalized populations.
To explore how pollen was transferred among the population, Tashiro was selected as a research area because the number of plants sampled there was the largest of the five areas. To analyze the S alleles of seeds, plant Ts6 was selected as representative. On plant Ts6 we recorded the internode lengths between siliques and the relative positions of seeds in the silique (Fig. 5B and 5C, Supplementary Fig. S1C). In the selected plant, one representative middle branch (branch D) was analyzed (Fig. 5B and 5C). The S alleles of the seeds on branch D were determined using DNA extracted from them (Fig. 5B). Interestingly, allele RsS23, which was detected in seed D2, was also in other plants within the 1 m × 1 m quadrat at Tashiro. In contrast, allele RsS12, which was detected in seeds D3, D4, D5, D6, D7, D10 and D11, was not present in other plants within the 1 m × 1 m quadrat at Tashiro. Therefore, the pollen with RsS12 appears to have come from a plant outside the 1 m × 1 m quadrat, and the pollen with RsS23 appears to have come from inside this range. The seeds D3, D4, D8 and D9 did not have allele RsS27, indicating that the other S allele, which was not determined in the plant Ts6, had been inherited. Another notable result was that three alleles, RsS8, RsS12 and RsS27, were detected in seed D1. RsS8 and RsS12 must have been inherited from the pollen because RsS27 was detected in plant Ts6. In wild-type Arabidopsis thaliana, another species in Brassicaceae, the frequency of spontaneous twin embryos is 0.02% (Vernon and Meinke, 1994). Therefore, the seed D1 may have been a twin produced by multiple independent fertilization, as occurs in Arabidopsis.
Phylogenetic analysis of SRK alleles in Brassica and Raphanus species
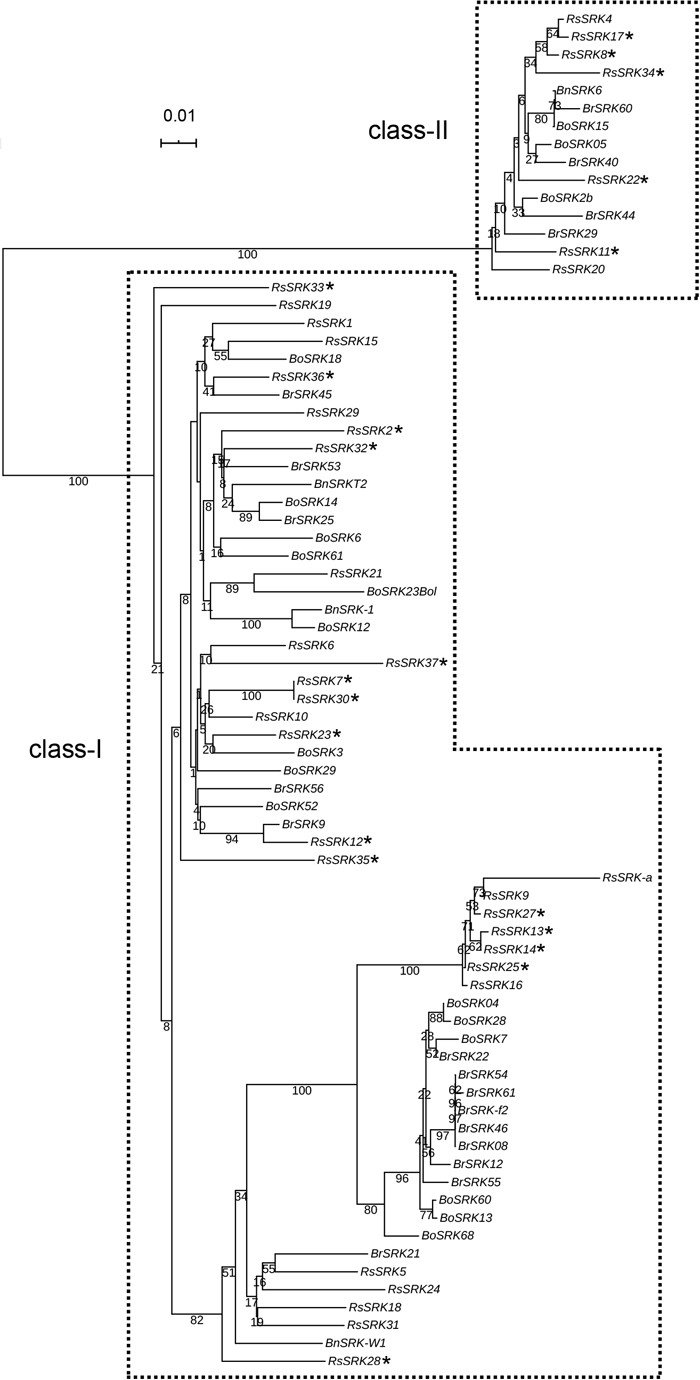
To examine the evolutionary relationships among the SRK alleles characterized in Yakushima and other SRK alleles of R. sativus, B. rapa, B. oleracea and B. napus, a phylogenetic tree was constructed using the neighbor-joining method with the sequences determined in this study and those already deposited in the databases (Fig. 6). Two clusters (class-I and class-II) were clearly identified. Furthermore, the class-I cluster was divided into two subclusters. Recently, from crystal structure analysis, although the SP11–SRK complex structure was found to be similar in BrS8 and BrS9, in B. rapa, the interactive regions between SP11 and SRK were different between BrS8 and BrS9 (Murase et al., 2020). The class-I SRK alleles of R. sativus were also classified into two different subclades (Fig. 6). In the class-I BrS9 subclade, the SRK alleles of each species were scattered within the cluster. However, in the class-I BrS8 subcluster and the class-II cluster, the SRK alleles were separated into a different clade in Brassica and Raphanus, except for a few alleles. Each cluster would have undergone different evolutionary processes. Interestingly, the SRK alleles identified in plants from Yakushima were found in all three of the clusters (class-I BrS8, class-I BrS9 and class-II), suggesting that the S alleles of the naturalized radish in Yakushima are highly diverse.
Our results from Yakushima show the S allele distribution in relation to positions of the plants in situ in naturalized radish populations for the first time and provide evidence that pollination occurs between neighboring plants within a population. Interestingly, novel SRK alleles were identified in the naturalized populations. There were differences in S allele distribution between the naturalized populations in the different sampling areas, suggesting that S allele diversity in the naturalized populations is maintained. Our methodology, combining S allele distribution and positions of the plants in situ, should be applicable to other self-incompatible species to dissect the diversity of the S allele distribution and provide insight into the ecological diversity of their genetics.







