ABSTRACT
Plaque vulnerability is associated with the degree of carotid artery stenosis (CS) and the risk of stroke. MicroRNAs (miRNAs) exert critical functions in disease progression, although only a few miRNAs have been well identified in CS. Therefore, this study aimed to investigate the differential expression profile of miRNAs and their potential functions in plaques of CS patients. Three CS patients with stable plaques and three patients with vulnerable plaques who underwent carotid endarterectomy were enrolled in this study. Differentially expressed miRNAs (DEmiRNAs) between patients with stable and vulnerable plaques were determined using small RNA sequencing. Target genes of DEmiRNAs were predicted and submitted to functional analyses. Validation of dysregulated DEmiRNAs was determined using quantitative real-time polymerase chain reaction (qRT-PCR). After sequencing, 76 DEmiRNAs were identified in vulnerable plaques, including 53 upregulated miRNAs and 23 downregulated miRNAs. Next, 23,495 target genes of the identified DEmiRNAs were predicted and functionally analyzed. This indicated that the target genes of the identified DEmiRNAs were mainly enriched in protein phosphorylation, transcription, nitrogen compound metabolism, endocytosis and autophagy, and related to signaling pathways of Hippo, MAPK, insulin, TGF-β, FoxO, AMPK and p53. Furthermore, qRT-PCR results for six miRNAs showed that five (83%) of them (hsa-miR-511-5p, hsa-miR-150-5p, hsa-miR-378a-5p, hsa-miR-365b-5p and hsa-miR-6511b-5p) were consistent with the sequencing results. Differential expression profiles and potential function of miRNAs associated with plaque stability in CS patients are identified for the first time, which should help to understand the regulatory mechanism of plaque stability in CS.
INTRODUCTION
Carotid artery stenosis (CS) is an atherosclerotic disease that affects extracranial carotid arteries, and is closely related to ipsilateral neurological events (Jeong et al., 2021; Krist et al., 2021). CS is thought to be responsible for approximately 30% of strokes (Wijeratne et al., 2020). The incidence of CS in people over 80 years old is about 5–7% in women, and 9–12% in men (Wijeratne et al., 2020). Currently, carotid endarterectomy and carotid stent implantation are the primary therapeutic strategies to prevent secondary stroke in cases where drug treatment is ineffective (Shimonaga et al., 2021). However, there is increasing evidence that plaque vulnerability is associated with the degree of stenosis in CS (Kurosaki et al., 2016; Shimonaga et al., 2021). Furthermore, carotid plaques undergo a remodeling process, and mild carotid stenosis sometimes leads to cerebrovascular events. Therefore, plaque characteristics, not just stenosis, may be critical for identifying high-risk groups for stroke (Kwee et al., 2008; Jeong et al., 2021). Thus, the present study focuses on carotid plaques, which is highly relevant to understanding CS pathogenesis.
MicroRNAs (miRNAs) are a class of small non-coding RNAs (18–25 nucleotides) and act as negative gene modulators to suppress the expression of target genes (Achkar et al., 2016). Accumulating evidence indicates that miRNAs exert critical roles in disease progression, including CS. For example, Jeong et al. (2021) reported miR-33a-5p, miR-33b-5p and miR-148a-3p as candidate biomarkers of CS because they bind directly to ATP binding cassette transporter 1 (ABCA1). Liu et al. (2021) showed that miR-28-5p alleviated CS by modulating vascular smooth muscle cell proliferation and migration. Zhang and Liu (2021) found that serum miR-637 was considerably reduced in CS patients, and its downregulation was a potential biomarker for the diagnosis of CS and a valuable predictor of cerebral ischemic events. Moreover, a relationship between miRNAs and plaque stability in CS has been reported recently. Wei et al. (2019) discovered that miR-330-5p was upregulated in symptomatic CS patients, which may cause carotid plaques to become unstable through binding of the miRNA to Talin-1. Luque et al. (2018) found that serum miR-638 in patients with high-grade CS was an independent predictor of plaque instability. Badacz et al. (2018) showed that plaque morphology and structure were associated with a change of miRNA levels in CS. While several miRNAs have been reported to function as regulators in plaque stability, there is a lack of research on screening the differential expression profile of miRNAs in plaques of CS patients.
Hence, this study focused on investigating the differential expression profile of miRNAs between stable and vulnerable plaques, and their potential function in CS patients, using small RNA sequencing, thereby clarifying the relationship between miRNAs and plaque stability.
RESULTS
Identification of DEmiRNAs between stable and vulnerable plaques
To study the relationship between miRNAs and plaque stability of CS, RNA-seq analysis was conducted to explore differential expression profiles of miRNAs between stable and vulnerable plaques of CS patients. We identified 76 DEmiRNAs between stable and vulnerable plaques. Among them, 53 were upregulated and 23 were downregulated in vulnerable plaques compared to stable ones (Fig. 1A, Table 1). Bidirectional clustering analysis indicated that these 76 DEmiRNAs could effectively differentiate the stable plaques from the vulnerable plaques (Fig. 1B).

Table 1. All differentially expressed miRNAs between stable plaques and vulnerable plaques
| miRNA_id | log2FC | pval | padj | regulation |
|---|
| hsa-miR-122-5p | −3.64004 | 0.000216 | 0.025508 | down |
| hsa-miR-365b-5p | −1.24265 | 0.042759 | 0.502474 | down |
| hsa-miR-6511b-5p | −1.9919 | 0.017396 | 0.320088 | down |
| hsa-miR-486-5p | −2.13339 | 0.000441 | 0.03649 | down |
| hsa-miR-206 | −3.12805 | 0.002124 | 0.09198 | down |
| hsa-miR-3158-3p | −1.70134 | 0.002333 | 0.09198 | down |
| hsa-miR-299-3p | −2.22142 | 0.004861 | 0.143736 | down |
| hsa-miR-1298-5p | −2.59352 | 0.006004 | 0.165718 | down |
| hsa-miR-3529-5p | −3.04541 | 0.007784 | 0.184149 | down |
| hsa-miR-4667-5p | −2.93377 | 0.010402 | 0.218973 | down |
| hsa-miR-302a-5p | −1.96812 | 0.019339 | 0.339052 | down |
| hsa-miR-6827-5p | −3.22707 | 0.019655 | 0.339052 | down |
| hsa-miR-302b-3p | −3.926 | 0.021645 | 0.355662 | down |
| hsa-miR-337-5p | −2.09735 | 0.021907 | 0.355662 | down |
| hsa-miR-411-5p | −1.25849 | 0.029119 | 0.416566 | down |
| hsa-miR-3124-5p | −1.91521 | 0.031542 | 0.416566 | down |
| hsa-miR-518a-3p | −5 | 0.038538 | 0.47839 | down |
| hsa-miR-6845-5p | −3.97728 | 0.03871 | 0.47839 | down |
| hsa-miR-370-5p | −2.22417 | 0.041273 | 0.502474 | down |
| hsa-miR-136-5p | −1.7742 | 0.042104 | 0.502474 | down |
| hsa-miR-134-5p | −1.71057 | 0.043747 | 0.502474 | down |
| hsa-miR-134-3p | −2.86876 | 0.0447 | 0.502474 | down |
| hsa-miR-193a-3p | −1.74326 | 0.046295 | 0.504376 | down |
| hsa-miR-150-5p | 3.003266 | 0.000176 | 0.024355 | up |
| hsa-miR-378a-5p | 3.123989 | 0.01434 | 0.269843 | up |
| hsa-miR-511-5p | 4.373096 | 1.23E-05 | 0.004337 | up |
| hsa-miR-5571-3p | 3.459432 | 2.40E-06 | 0.001985 | up |
| hsa-miR-511-3p | 4.198021 | 1.57E-05 | 0.004337 | up |
| hsa-miR-139-5p | 3.544346 | 0.000134 | 0.024355 | up |
| hsa-miR-223-3p | 2.836228 | 0.000173 | 0.024355 | up |
| hsa-miR-139-3p | 3.357306 | 0.000349 | 0.036159 | up |
| hsa-miR-181a-3p | 2.647194 | 0.000432 | 0.03649 | up |
| hsa-miR-6503-3p | 2.68362 | 0.000725 | 0.054565 | up |
| hsa-miR-4662a-5p | 4.207158 | 0.00106 | 0.072307 | up |
| hsa-miR-424-5p | 2.543496 | 0.001135 | 0.072307 | up |
| hsa-miR-126-3p | 3.037815 | 0.00171 | 0.09198 | up |
| hsa-miR-223-5p | 2.860742 | 0.00178 | 0.09198 | up |
| hsa-miR-146b-5p | 2.492507 | 0.001957 | 0.09198 | up |
| hsa-miR-10b-3p | 3.405553 | 0.002051 | 0.09198 | up |
| hsa-miR-142-3p | 1.847459 | 0.002122 | 0.09198 | up |
| hsa-miR-126-5p | 2.860106 | 0.002256 | 0.09198 | up |
| hsa-miR-31-3p | 6.087463 | 0.002538 | 0.095505 | up |
| hsa-miR-3614-3p | 5.141012 | 0.002869 | 0.09947 | up |
| hsa-miR-944 | 3.982722 | 0.002976 | 0.09947 | up |
| hsa-miR-424-3p | 2.952956 | 0.003003 | 0.09947 | up |
| hsa-miR-6503-5p | 2.401473 | 0.004164 | 0.1326 | up |
| hsa-miR-10a-5p | 2.541213 | 0.004819 | 0.143736 | up |
| hsa-miR-450a-5p | 2.363606 | 0.005179 | 0.147865 | up |
| hsa-miR-212-5p | 2.716938 | 0.006587 | 0.175936 | up |
| hsa-miR-542-3p | 1.916604 | 0.007062 | 0.182731 | up |
| hsa-miR-3690 | 2.416236 | 0.007382 | 0.184149 | up |
| hsa-miR-142-5p | 1.918706 | 0.007595 | 0.184149 | up |
| hsa-miR-10a-3p | 2.670799 | 0.008157 | 0.187612 | up |
| hsa-miR-10b-5p | 2.69921 | 0.008512 | 0.190492 | up |
| hsa-miR-150-3p | 2.462143 | 0.01044 | 0.218973 | up |
| hsa-miR-216b-5p | 4.1105 | 0.010578 | 0.218973 | up |
| hsa-miR-130b-5p | 2.327165 | 0.011373 | 0.229677 | up |
| hsa-miR-1247-3p | 5.087463 | 0.012294 | 0.242377 | up |
| hsa-miR-450b-5p | 2.031722 | 0.013085 | 0.251953 | up |
| hsa-miR-653-3p | 3.402098 | 0.019176 | 0.339052 | up |
| hsa-miR-340-3p | 1.855457 | 0.020151 | 0.340513 | up |
| hsa-miR-132-3p | 1.655059 | 0.022978 | 0.36142 | up |
| hsa-miR-625-5p | 5.01495 | 0.023134 | 0.36142 | up |
| hsa-miR-222-3p | 1.795496 | 0.024649 | 0.377948 | up |
| hsa-miR-224-5p | 1.609181 | 0.028889 | 0.416566 | up |
| hsa-miR-99a-5p | 1.304528 | 0.0293 | 0.416566 | up |
| hsa-miR-873-5p | 3.763766 | 0.029354 | 0.416566 | up |
| hsa-miR-1273h-3p | 2.843984 | 0.030354 | 0.416566 | up |
| hsa-miR-3910 | 4.123382 | 0.030465 | 0.416566 | up |
| hsa-miR-146b-3p | 1.566823 | 0.031634 | 0.416566 | up |
| hsa-miR-3934-5p | 1.725183 | 0.031695 | 0.416566 | up |
| hsa-miR-6502-5p | 1.95996 | 0.032267 | 0.417455 | up |
| hsa-miR-3064-3p | 4.857981 | 0.033603 | 0.428055 | up |
| hsa-miR-199b-5p | 1.67698 | 0.044313 | 0.502474 | up |
| hsa-miR-3613-5p | 1.482611 | 0.044907 | 0.502474 | up |
| hsa-miR-499a-5p | 1.379631 | 0.045929 | 0.504376 | up |
To clarify the functions of the identified DEmiRNAs in plaque stability in CS, a total of 23,495 of their target genes were first predicted, and then submitted to functional analyses. The gene ontology (GO) terms analyzed included biological process (BP), cellular component (CC) and molecular function (MF). In the BP terms, the predicted target genes of the identified DEmiRNAs were significantly enriched in “protein phosphorylation”, “transcription, DNA-templated”, “nitrogen compound metabolic process”, “cytoskeleton organization” and “multicellular organism development” (Fig. 2A). In the CC analysis, the target genes were mainly enriched in “nucleus”, “Golgi apparatus”, “cytosol”, “endomembrane system” and “organelle membrane” (Fig. 2B). Furthermore, MF analysis showed that the target genes of the identified DEmiRNAs were involved in “nucleic acid binding”, “DNA binding”, “catalytic activity”, “ATP binding” and “cytoskeletal protein binding” (Fig. 2C).
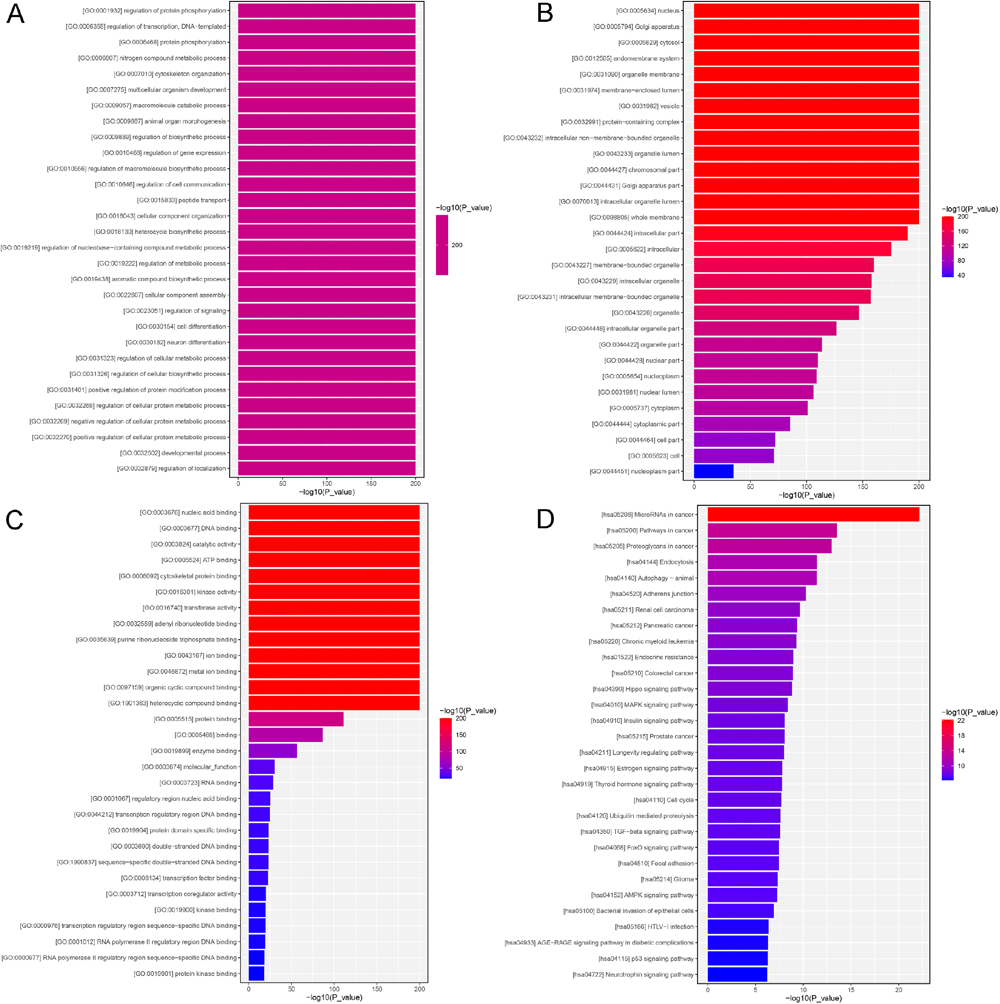
In addition, KEGG (Kyoto Encyclopedia of Genes and Genomes) pathway enrichment analysis revealed that these target genes of the identified DEmiRNAs were closely related to endocytosis, autophagy and adherens junction processes, and associated with the signaling pathways of Hippo, MAPK, insulin, TGF-β, FoxO, AMPK and p53 (Fig. 2D).
Validation of dysregulated DEmiRNAs in CS
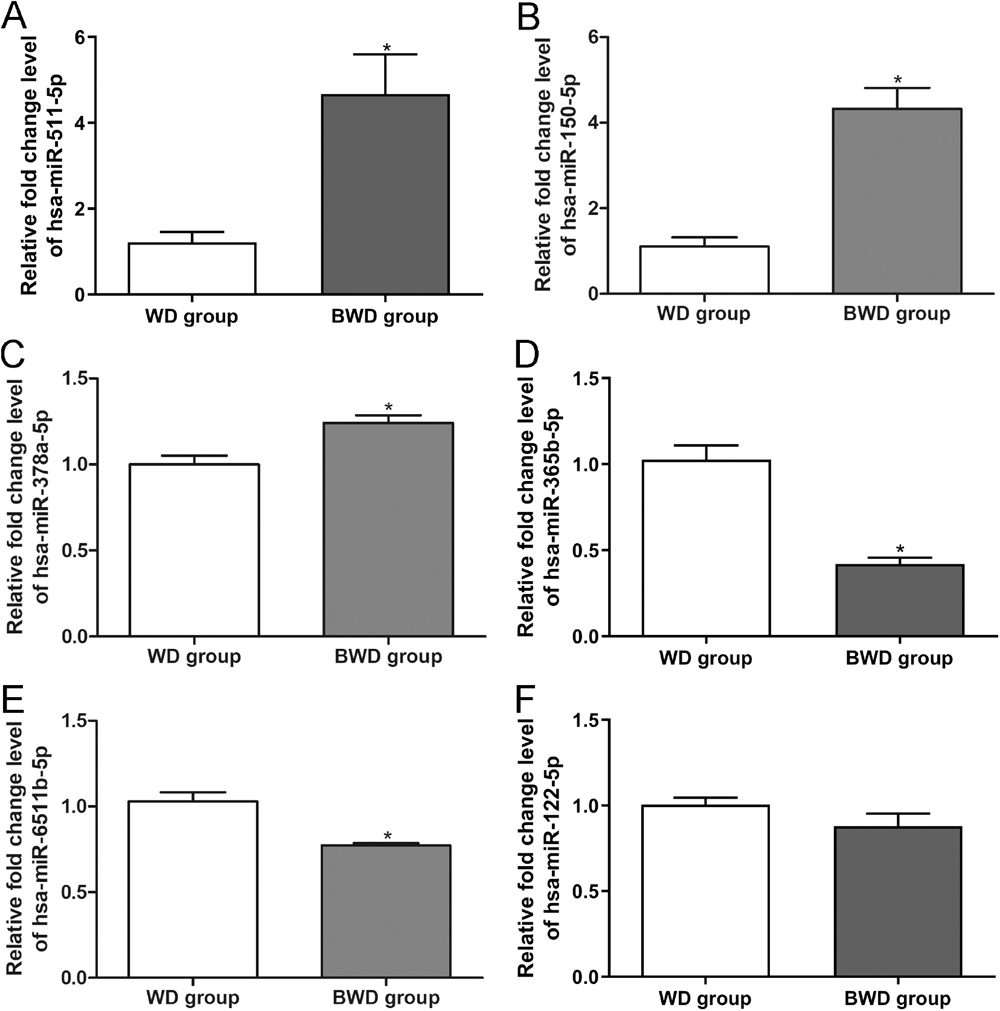
To verify the small RNA sequencing results, three significantly upregulated DEmiRNAs (hsa-miR-511-5p, hsa-miR-150-5p, hsa-miR-378a-5p) and three significantly downregulated DEmiRNAs (hsa-miR-365b-5p, hsa-miR-122-5p, hsa-miR-6511b-5p) were chosen for quantitative real-time polymerase chain reaction (qRT-PCR) assay. The relative fold change levels of hsa-miR-511-5p, hsa-miR-150-5p and hsa-miR-378a-5p were significantly higher in vulnerable plaques compared to the stable plaques (P < 0.05, Fig. 3A–3C). Furthermore, the relative fold change levels of hsa-miR-365b-5p and hsa-miR-6511b-5p were significantly lower in vulnerable plaques than in stable plaques (P < 0.05, Fig. 3D and 3E). However, there was no significant difference in the relative fold change level of hsa-miR-122-5p between the two groups (Fig. 3F). The results indicated that the concordance rate of the sequencing results and qRT-PCR outcomes was approximately 83%, which showed the high reliability of small RNA sequencing.
DISCUSSION
Carotid artery stenosis (CS) is an atherosclerotic disease that may lead to ipsilateral neurological events such as strokes, and is thus an important threat to global health (Wijeratne et al., 2020; Jeong et al., 2021; Krist et al., 2021). In recent years, plaque vulnerability has been proven to be related to the degree of stenosis in CS and the risk of stroke occurrence (Kwee et al., 2008; Kurosaki et al., 2016; Jeong et al., 2021; Shimonaga et al., 2021). Furthermore, as critical regulators at the post-transcriptional level, some miRNAs have been reported to participate in the regulation of plaque stability and characteristics (Badacz et al., 2018; Luque et al., 2018; Wei et al., 2019). However, there has been no comprehensive study on the expression and function of miRNAs in CS plaque stability up to now. Therefore, this study elaborates the expression profile and potential function of miRNAs in plaques of CS.
We identified 76 DEmiRNAs between stable and vulnerable plaques of CS patients. GO analysis revealed that the target genes of these DEmiRNAs mainly participated in the modulation of protein phosphorylation, DNA-templated transcription and nitrogen compound metabolic process. Several studies have proved that protein phosphorylation is involved in regulating plaque stability. For example, Sigala et al. (2017) showed that eNOS phosphorylation was increased in stable plaques compared with unstable plaques. Furthermore, Zhang et al. (2020) revealed via bioinformatics analysis that DEmiRNAs in unstable atherosclerotic plaques were also mainly enriched in the regulation of DNA-templated transcription. Moreover, previous studies demonstrated that nitrogen compound metabolism plays regulatory roles in plaque stability (Depre et al., 1999; Ning et al., 2020). Ning et al. (2020) found that the restored balance of the NOS-NO system contributed to the plaque-stabilizing effect of Leonurine. Depre et al. (1999) suggested that iNOS expression is induced in coronary atherosclerotic plaques and associated with different factors of instability. Therefore, biological processes enriched by targeted genes of the identified DEmiRNAs including protein phosphorylation, transcription and nitrogen metabolism may be critical for plaque vulnerability.
KEGG analysis showed that the target genes of DEmiRNAs were mainly related to endocytosis and autophagy, as well as the signaling pathways of Hippo, MAPK, insulin, TGF-β, FoxO, AMPK and p53. Low-density lipoprotein (LDL) level is a risk factor for CS; LDL enters cells mainly through endocytosis (Wattis et al., 2008; Araki et al., 2012). Besides, Liu et al. (2015) found that autophagy was activated in smooth muscle cells, endothelial cells and macrophages in unstable atherosclerotic plaques. Sigala et al. (2003) found that the adherens junction protein VE-cadherin in carotid plaques is linked with plaque instability, a high degree of stenosis, and clinical events. Another study reported that the Hippo pathway plays an essential role in developing the cardiovascular system and maintaining vessel homeostasis in cardiovascular diseases (He et al., 2018). Furthermore, Zhang et al. (2013) demonstrated that inhibition of the MAPK signaling pathway enhanced plaque stability. Interestingly, a previous paper demonstrated that defects in the insulin signaling pathway resulted in cardiovascular disease (Morisco et al., 2006). Inhibition of TGF-β signaling accelerates plaque formation and progression toward an unstable phenotype (Cipollone et al., 2004). Shimba et al. (2021) showed that overexpression of FoxO-1 led to a 65% reduction of atherosclerotic plaque area in ApoE-KO mice, while Xiong et al. (2020) found that the AMPK signaling pathway mediated the protective effects of atorvastatin on atherosclerotic plaque formation and plaque stability. Moreover, the p53 signaling pathway has been shown to regulate vascular remodeling and atherosclerotic plaque formation (Mayr et al., 2002). Taking these findings together, the functions of endocytosis and autophagy, and of the Hippo, MAPK, insulin, TGF-β, FoxO, AMPK and p53 signaling pathways, may have an essential role in plaque stability, which implies that the differentially expressed miRNAs screened in this study have considerable research potential. However, the detailed mechanisms of these pathways in plaque stability of CS need to be further clarified.
In addition, three upregulated and three downregulated DEmiRNAs were selected for qRT-PCR verification, and we found that hsa-miR-511-5p, hsa-miR-150-5p and hsa-miR-378a-5p were significantly upregulated, whereas hsa-miR-365b-5p and hsa-miR-6511b-5p were downregulated, in vulnerable plaques. hsa-miR-511-5p has been reported to present abnormal expression in coronary artery disease (Zhong et al., 2018). However, its role in the progression of coronary artery disease is unclear. Previous studies indicated that inhibition of hsa-miR-150-5p modulated vascular integrity, inflammation, endothelial cell apoptosis, plaque stabilization and progression of atherosclerosis (Qin et al., 2017; Gong et al., 2018; Rozhkov et al., 2022). Liu et al. (2019) demonstrated that hsa-miR-378a-5p regulated proliferation and migration of vascular smooth muscle cells via binding to CDK1 in CS. A previous study (Sun et al., 2022) showed that miR-6511b-5p could inhibit the invasion and migration of colorectal cancer cells by directly targeting CD44 methylation of BRG1, thereby acting as a promising biomarker and treatment target for metastatic colorectal cancer. Another study clarified that miR-365b-3p could suppress the proliferation and migration of human coronary smooth muscle cells by directly targeting ADAMTS1 in coronary atherosclerosis. Combining these and our results, it can be inferred that the plaque instability of CS is associated with the upregulation of hsa-miR-511-5p, hsa-miR-150-5p and hsa-miR-378a-5p, and the downregulation of hsa-miR-365b-5 and hsa-miR-6511b-5p. However, the specific roles of these miRNAs and other identified DEmiRNAs in plaque stability of CS are still unknown; therefore, the function and molecular mechanisms of these DEmiRNAs warrant further investigation.
In conclusion, the differential expression profile of miRNAs associated with plaque stability of CS patients, and their potential function, have been clarified for the first time. This comprehensive analysis deepens our understanding of plaque stability in CS, and provides a theoretical basis for miRNAs to be used to prevent or diagnose plaque rupture.
MATERIALS AND METHODS
Collection of clinical samples
Three CS patients with stable plaques (WD group) and three patients with vulnerable plaques (BWD group) who underwent carotid endarterectomy at Shanghai Pudong Hospital were enrolled in this study. All the patients received carotid vascular ultrasonography and carotid contrast-enhanced ultrasound examination and were diagnosed with severe lumen stenosis (stenosis rate 70–99%). Excised plaque samples were stored at −80 ℃ until further use. The Ethics Committee of Shanghai Pudong Hospital approved the study, and informed consent was given by all the patients.
RNA extraction and small RNA sequencing
Total RNA was extracted from all the plaque samples using TRIzol (Invitrogen, CA, USA) following the supplier’s protocols. The concentration and quality of the isolated RNA were determined using a NanoDrop 2000 (Thermo Fisher Scientific, MA, USA). Sequencing libraries were then constructed using the NEBNext Ultra Directional RNA Library Prep Kit for Illumina (New England Biolabs, MA, USA) based on the supplier’s protocols. Briefly, the purified RNA samples were ligated with 3′ and 5′ adapters, followed by RT-PCR to generate cDNA. After that, the purified cDNA library products were used to perform single-end sequencing on an Illumina Hiseq2500 at Yanzai Biotechnology (Shanghai, China).
Bioinformatic analysis
Raw data were used for quality control using FastqStat.jar V1.0, and clean data were obtained. These data were then annotated based on the Rfam database (11.0) by BLAST software V2.3.0, and mapped to the human reference genome based on the miRBase database by Bowtie software V1.2.1.1. Subsequently, the expression levels of known miRNAs in each sample were statistically analyzed, and normalized as transcripts per million using RNAfold V2.1.8 and miRDeep2 V0.1.0. Next, DEGseq2 software was used to screen the differentially expressed miRNA (DEmiRNAs) between stable plaques and vulnerable plaques, with the thresholds of P < 0.05 and |log2fold change (FC)| > 1. Target genes for the identified DEmiRNAs were predicted using the miRDB (http://mirdb.org/) and TargetScan (http://www.targetscan.org/) databases. Finally, these target genes were submitted to functional analyses based on GO and KEGG, and the DAVID database V 6.8 was applied to visualize significantly enriched pathways (P < 0.05).
qRT-PCR
Relative fold change levels of the selected DEmiRNAs were determined using a stem-loop method. Briefly, total RNA was reverse transcribed into cDNA using the PrimeScript II 1st Strand cDNA Synthesis Kit (TaKaRa, Tokyo, Japan) according to the manufacturer’s instructions. Briefly, a 20-μl mixture was prepared using 3 μl RT-Primer (10 μM), 1 μl dNTP Mixture (10 mM), 300 ng RNA and RNase Free H2O and incubated at 65 ℃ for 5 min. A 10-μl aliquot was then mixed with 4 μl 5×PrimeScript II Buffer, 0.5 μl RNase Inhibitor (40 U/μl), 1 μl PrimeScript II RTase (200 U/μl) and 4.5 μl RNase Free H2O. The reaction was incubated at 42 ℃ for 60 min, and finished at 95 ℃ for 5 min. The qRT-PCR assay was conducted using Power SYBR Green PCR Master Mix (Thermo Fisher Scientific) following the supplier’s protocols. U6 was used as a housekeeping gene, and the relative fold change levels of the selected DEmiRNAs were calculated by the 2−ΔΔCt method. The primer sequences are shown in Table 2.
Table 2. Sequences of all primers
| Primer | Sequence (5′–3′) |
|---|
| Downstream universal primer | GTGCAGGGTCCGAGGT |
| hsa-U6-RT | GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACAAAATATG |
| hsa-U6-F | CTCGCTTCGGCAGCACA |
| hsa-U6-R | AACGCTTCACGAATTTGCGT |
| hsa-miR-511-5p-JH | GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACTGACTG |
| hsa-miR-511-5p -F | GCCGGTGTCTTTTGCTCTG |
| hsa-miR-150-5p-JH | GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACCACTGG |
| hsa-miR-150-5p-F | GCCGTCTCCCAACCCTTGTA |
| hsa-miR-378a-5p-JH | GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACACACAG |
| hsa-miR-378a-5p-F | GCGCTCCTGACTCCAGGTC |
| hsa-miR-365b-5p-JH | GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACACAGCT |
| hsa-miR-365b-5p-F | GCAGGGACTTTCAGGGGC |
| hsa-miR-122-5p-JH | GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACCAAACA |
| hsa-miR-122-5p-F | GCCGTGGAGTGTGACAATGG |
| hsa-miR-6511b-5p-JH | GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACTGTCAG |
| hsa-miR-6511b-5p-F | GCTGCAGGCAGAAGTGGGG |
Data are described as mean ± standard deviation (SD), and statistical analysis was conducted using GraphPad Prism 7. Student’s t-test was utilized to evaluate differences between two groups. P < 0.05 was considered a significant difference.
FUNDING
This study was supported by the Pudong New Area Clinical Plateau Discipline Project (No. PWYgy2021-03), the New and Cross-disciplinary Construction of Shanghai Pudong New Area Health Commission (No. PWXx2020-01), a Scientific Research Project of Shanghai Municipal Health Commission (No. 202150004) and “A study on multi-dimensional evaluation of carotid stenosis risk and prediction of cerebral function after revascularization using SSPC system” (No. KP9202102).
COMPETING INTERESTS
The authors declare that they have no competing interests.
REFERENCES
- Achkar, N. P., Cambiagno, D. A., and Manavella, P. A. (2016) miRNA biogenesis: a dynamic pathway. Trends Plant Sci. 21, 1034–1044.
- Araki, Y., Kumakura, H., Kanai, H., Kasama, S., Sumino, H., Ichikawa, A., Ito, T., Iwasaki, T., Takayama, Y., Ichikawa, S., et al. (2012) Prevalence and risk factors for cerebral infarction and carotid artery stenosis in peripheral arterial disease. Atherosclerosis 223, 473–477.
- Badacz, R., Przewłocki, T., Gacoń, J., Stępień, E., Enguita, F. J., Karch, I., Żmudka, K., and Kabłak-Ziembicka, A. (2018) Circulating miRNA levels differ with respect to carotid plaque characteristics and symptom occurrence in patients with carotid artery stenosis and provide information on future cardiovascular events. Postepy Kardiol. Interwencyjnej 14, 75–84.
- Cipollone, F., Fazia, M., Mincione, G., Iezzi, A., Pini, B., Cuccurullo, C., Ucchino, S., Spigonardo, F., Di Nisio, M., Cuccurullo, F., et al. (2004) Increased expression of transforming growth factor-β1 as a stabilizing factor in human atherosclerotic plaques. Stroke 35, 2253–2257.
- Depre, C., Havaux, X., Renkin, J., Vanoverschelde, J. L. J., and Wijns, W. (1999) Expression of inducible nitric oxide synthase in human coronary atherosclerotic plaque. Cardiovasc. Res. 41, 465–472.
- Gong, F.-H., Cheng, W.-L., Wang, H., Gao, M., Qin, J.-J., Zhang, Y., Li, X., Zhu, X., Xia, H., and She, Z.-G. (2018) Reduced atherosclerosis lesion size, inflammatory response in miR-150 knockout mice via macrophage effects. J. Lipid Res. 59, 658–669.
- He, J., Bao, Q., Yan, M., Liang, J., Zhu, Y., Wang, C., and Ai, D. (2018) The role of Hippo/yes-associated protein signalling in vascular remodelling associated with cardiovascular disease. Br. J. Pharmacol. 175, 1354–1361.
- Jeong, S., Jun, J. H., Kim, J. Y., Park, H. J., Cho, Y.-P., and Kim, G. J. (2021) Expression of miRNAs targeting ATP binding cassette transporter 1 (ABCA1) among patients with significant carotid artery stenosis. Biomedicines 9, 920.
- Krist, A. H., Davidson, K. W., Mangione, C. M., Barry, M. J., Cabana, M., Caughey, A. B., Donahue, K., Doubeni, C. A., Epling, J. W., Kubik, M., et al. (2021) Screening for asymptomatic carotid artery stenosis: US preventive services Task force recommendation statement. JAMA 325, 476–481.
- Kurosaki, Y., Yoshida, K., Fukumitsu, R., Sadamasa, N., Handa, A., Chin, M., and Yamagata, S. (2016) Carotid artery plaque assessment using quantitative expansive remodeling evaluation and MRI plaque signal intensity. J. Neurosurg. 124, 736–742.
- Kwee, R. M., van Oostenbrugge, R. J., Hofstra, L., Teule, G. J., van Engelshoven, J. M., Mess, W. H., and Kooi, M. E. (2008) Identifying vulnerable carotid plaques by noninvasive imaging. Neurology 70, 2401–2409.
- Liu, H., Cao, Y., Tong, T., Shi, J., Zhang, Y., Yang, Y., and Liu, C. (2015) Autophagy in atherosclerosis: a phenomenon found in human carotid atherosclerotic plaques. Chin. Med. J. 128, 69–74.
- Liu, Q., Yan, S., Yuan, Y., Ji, S., and Guo, L. (2021) miR-28-5p improved carotid artery stenosis by regulating vascular smooth muscle cell proliferation and migration. Vascular 30, 764–770.
- Liu, S., Yang, Y., Jiang, S., Xu, H., Tang, N., Lobo, A., Zhang, R., Liu, S., Yu, T., and Xin, H. (2019) MiR-378a-5p regulates proliferation and migration in vascular smooth muscle cell by targeting CDK1. Front. Genet. 10, 22.
- Luque, A., Farwati, A., Krupinski, J., and Aran, J. M. (2018) Association between low levels of serum miR-638 and atherosclerotic plaque vulnerability in patients with high-grade carotid stenosis. J. Neurosurg. 131, 72–79.
- Mayr, U., Mayr, M., Li, C., Wernig, F., Dietrich, H., Hu, Y., and Xu, Q. (2002) Loss of p53 accelerates neointimal lesions of vein bypass grafts in mice. Circ. Res. 90, 197–204.
- Morisco, C., Lembo, G., and Trimarco, B. (2006) Insulin resistance and cardiovascular risk: new insights from molecular and cellular biology. Trends Cardiovasc Med. 16, 183–188.
- Ning, K., Wang, M.-J., Lin, G., Zhang, Y.-L., Li, M.-Y., Yang, B.-F., Chen, Y., Huang, Y., Li, Z.-M., Huang, Y.-J., et al. (2020) eNOS-nitric oxide system contributes to a novel antiatherogenic effect of leonurine via inflammation inhibition and plaque stabilization. J. Pharmacol. Exp. Ther. 373, 463–475.
- Qin, B., Shu, Y., Xiao, L., Lu, T., Lin, Y., Yang, H., and Lu, Z. (2017) MicroRNA-150 targets ELK1 and modulates the apoptosis induced by ox-LDL in endothelial cells. Mol. Cell. Biochem. 429, 45–58.
- Rozhkov, A. N., Shchekochikhin, D. Y., Ashikhmin, Y. I., Mitina, Y. O., Evgrafova, V. V., Zhelankin, A. V., Gognieva, D. G., Akselrod, A. S., and Kopylov, P. Y. (2022) The profile of circulating blood microRNAs in outpatients with vulnerable and stable atherosclerotic plaques: associations with cardiovascular risks. Noncoding RNA 8, 47.
- Shimba, Y., Senda, R., Katayama, K., Morita, A., Ikeda, M., Kamei, Y., and Miura, S. (2021) Skeletal muscle-specific forkhead box protein-O1 overexpression suppresses atherosclerosis progression in apolipoprotein E-knockout mice. Biochem. Biophys. Res. Commun. 540, 61–66.
- Shimonaga, K., Matsushige, T., Takahashi, H., Hashimoto, Y., Yoshiyama, M., Ono, C., and Sakamoto, S. (2021) Peptidylarginine deiminase 4 as a possible biomarker of plaque instability in carotid artery stenosis. J. Stroke Cerebrovasc. Dis. 30, 105816.
- Sigala, F., Efentakis, P., Karageorgiadi, D., Filis, K., Zampas, P., Iliodromitis, E. K., Zografos, G., Papapetropoulos, A., and Andreadou, I. (2017) Reciprocal regulation of eNOS, H2S and CO-synthesizing enzymes in human atheroma: correlation with plaque stability and effects of simvastatin. Redox Biol. 12, 70–81.
- Sigala, F., Vourliotakis, G., Georgopoulos, S., Kavantzas, N., Papalambros, E., Agapitos, M., and Bastounis, E. (2003) Vascular endothelial cadherin expression in human carotid atherosclerotic plaque and its relationship with plaque morphology and clinical data. Eur. J. Vasc. Endovasc. Surg. 26, 523–528.
- Sun, J., Ye, L., Shi, Y., Wang, X., Zhao, X., Ren, S., Fan, J., Shao, H., and Qin, B. (2022) MiR-6511b-5p suppresses metastasis of pMMR colorectal cancer through methylation of CD44 by directly targeting BRG1. Clin. Transl. Oncol. 24, 1940–1953.
- Wattis, J. A. D., O’Malley, B., Blackburn, H., Pickersgill, L., Panovska, J., Byrne, H. M., and Jackson, K. G. (2008) Mathematical model for low density lipoprotein (LDL) endocytosis by hepatocytes. Bull. Math. Biol. 70, 2303–2333.
- Wei, X., Sun, Y., Han, T., Zhu, J., Xie, Y., Wang, S., Wu, Y., Fan, Y., Sun, X., Zhou, J., et al. (2019) Upregulation of miR-330-5p is associated with carotid plaque’s stability by targeting Talin-1 in symptomatic carotid stenosis patients. BMC Cardiovasc. Disord. 19, 149.
- Wijeratne, T., Menon, R., Sales, C., Karimi, L., and Crewther, S. (2020) Carotid artery stenosis and inflammatory biomarkers: the role of inflammation-induced immunological responses affecting the vascular systems. Ann. Transl. Med. 8, 1276.
- Xiong, W., Fei, M., Wu, C., Wang, W., Luo, R., Shen, L., and Zhang, Z. (2020) Atorvastatin inhibits endoplasmic reticulum stress through AMPK signaling pathway in atherosclerosis in mice. Exp. Ther. Med. 19, 2266–2272.
- Zhang, K., Meng, X., Kong, J., Liu, F.-F., Yang, J.-M., Gao, F., Zhang, Y., and Zhang, C. (2013) Simvastatin increases Prolyl-4-Hydroxylase α1 expression in atherosclerotic plaque and ox-LDL-stimulated human aortic smooth muscle cells via p38 MAPK and ERK1/2 signaling. J. Mol. Cell. Cardiol. 65, 43–50.
- Zhang, R., Ji, Z., Yao, Y., Zuo, W., Yang, M., Qu, Y., Su, Y., Ma, G., and Li, Y. (2020) Identification of hub genes in unstable atherosclerotic plaque by conjoint analysis of bioinformatics. Life Sci. 262, 118517.
- Zhang, T., and Liu, R. (2021) Dysregulation of miR-637 serves as a diagnostic biomarker in patients with carotid artery stenosis and predicts the occurrence of the cerebral ischemic event. Bioengineered 12, 8658–8665.
- Zhong, Z., Hou, J., Zhang, Q., Zhong, W., Li, B., Li, C., Liu, Z., Yang, M., and Zhao, P. (2018) Circulating microRNA expression profiling and bioinformatics analysis of dysregulated microRNAs of patients with coronary artery disease. Medicine (Baltimore) 97, e11428.