2024 Volume 99 Article ID: 23-00287
2024 Volume 99 Article ID: 23-00287
The developmental mechanisms of limb buds have been studied in developmental biology as an excellent model of pattern formation. Chick embryos have contributed to the discovery of new principles in developmental biology, as it is easy to observe live embryos and manipulate embryonic tissues. Herein, I outline recent findings and future issues over the next decade regarding three themes, based on my research: limb positioning, proximal–distal limb elongation and digit identity determination. First, how hindlimb position is determined at the molecular level is described, with a focus on the transforming growth factor-β signaling molecule GDF11. Second, I explain how the cell population in the limb bud deforms with developmental progress, shaping the limb bud with elongation along the proximal–distal axis. Finally, I describe the developmental mechanisms that determine digit identity through the interdigits.
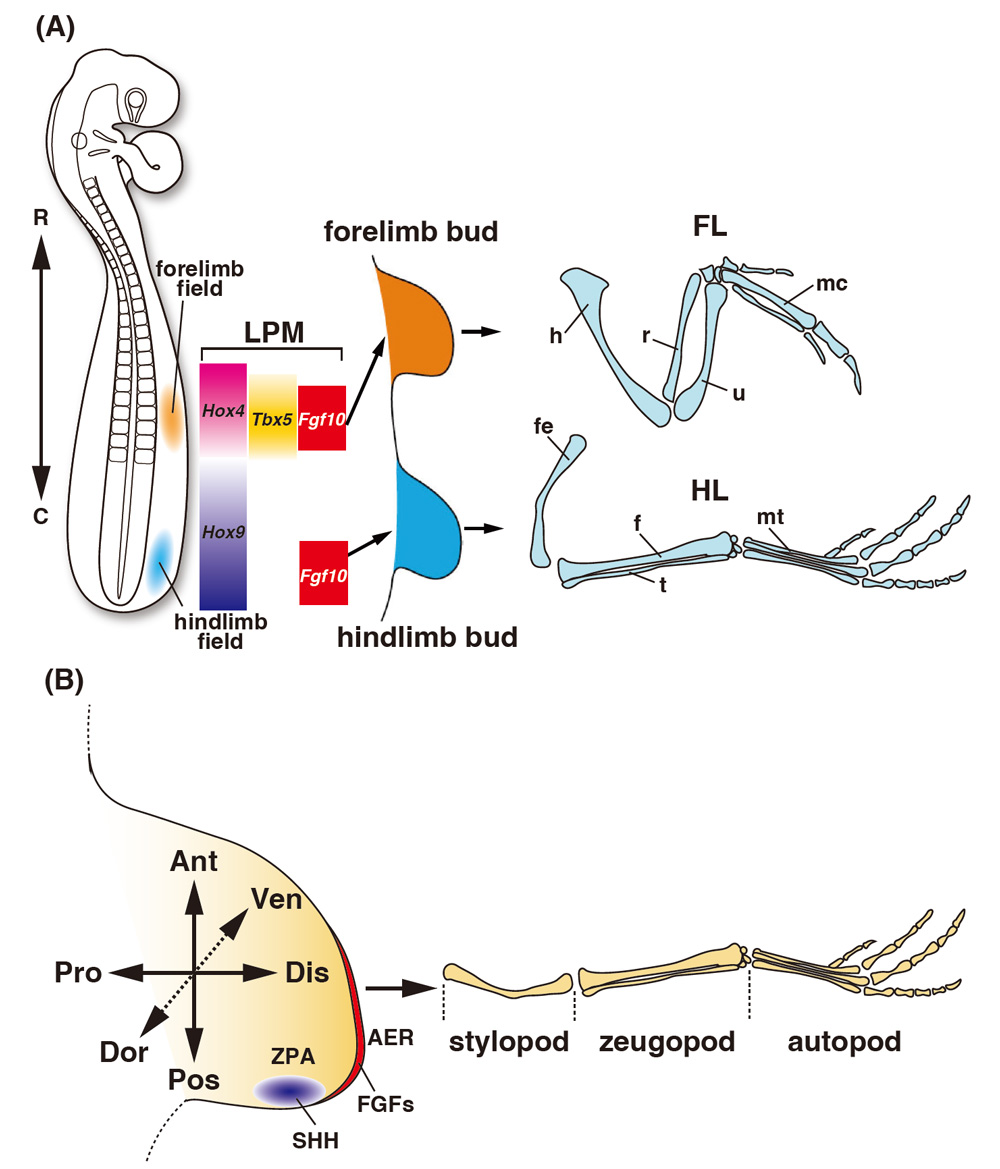
Vertebrates, including humans, have four limbs that protrude from the body. Limbs are formed along the anterior–posterior axis of the body during development. The limbs closer to the head are called forelimbs, whereas those farther from the head are called hindlimbs (Fig. 1A). During development, forelimbs and hindlimbs are formed at a somite (the primordium of the vertebral bone) position that is species-specific. In chicken embryos, the forelimb bud, which is the primordium of the forelimb, develops at the 16–21 somite level, whereas the hindlimb bud, which is the primordium of the hindlimb, develops at the 27–33 somite level (Matsubara et al., 2017). The limb bud emerges from a structure that proliferating lateral plate mesoderm cells cover with ectoderm, which becomes the future epidermis. The limb bud has three directional axes: the anterior–posterior axis from the thumb to the little finger, the proximal–distal axis from the trunk to the fingertips, and the dorsal–ventral axis from the back of the hand to the palm (Fig. 1B). Two main signaling centers maintain limb bud growth: the apical ectodermal ridge (AER), located at the distal end of the limb, and the zone of polarizing activity (ZPA), located on the posterior side of the limb bud. Fibroblast growth factors (FGFs) are expressed in the AER, and sonic hedgehog (SHH) is expressed in the ZPA. A positive feedback loop between FGFs and SHH maintains limb growth (Fig. 1B). After the limb bud is formed, it grows distally and forms a cartilaginous pattern. Based on this skeletal pattern, the limb buds are anatomically divided into stylopod, zugopod and autopod regions with increasing distance from the trunk (Fig. 1B). The humerus and femur are formed on the forelimbs and hindlimbs of the stylopod, the ulna/radius and fibula/tibia are formed on the zeugopod, and the metacarpal and metatarsal are formed on the autopod. Although the shape of the limbs differs in tetrapods, the basic pattern of limb bud formation is conserved across species.
In this review, I outline recent findings and future issues over the next decade regarding three themes: limb positioning, proximal–distal limb elongation and digit identity determination.
The position at which limb buds are formed along the anterior–posterior axis of the body differs depending on the species; however, the formation of both forelimb and hindlimb buds begins when the cells of the lateral plate mesoderm form a bulge. The positions of the limb buds are determined prior to budding. Limb bud development during budding is called limb initiation (Royle et al., 2021). In the region where forelimb buds are formed, the transcription factor Tbx5 is expressed in a slightly wider region before and after the prospective forelimb bud region, and the expression of Fgf10 is induced in the central part of the expression region of Tbx5 to initiate budding (Ohuchi et al., 1997; Takeuchi et al., 1999). TBX5 binds directly to the Fgf10 promoter and Tbx5 knockout mice do not induce Fgf10 expression (Agarwal et al., 2003). In zebrafish and chick embryos Wnt2b is also expressed in the Tbx5 region, suggesting that Wnt2b is required for Tbx5 expression (Ng et al., 2002). The involvement of the HoxC cluster, comprising nine genes encoding the Hox transcription factor, has been reported as a mechanism for localizing the expression of Tbx5, which is essential for forelimb bud initiation, around the prospective forelimb region (Nishimoto et al., 2014) (Fig. 1A). The HoxC cluster shows a nested expression pattern in somites along the anterior–posterior axis of the body. A lower number of Hox genes (e.g., HoxC4) is expressed up to the anterior side of the somites and a higher number of Hox genes (e.g., HoxC13) are expressed only on the posterior side. The Hox genes show a nested expression pattern in the lateral plate mesoderm and somites. According to Nishimoto et al. (2014), Tbx5 expression was suppressed when HoxC9 was introduced by electroporation into the lateral plate mesoderm of the prospective forelimb region in chick embryos. Their study suggested that HoxC9 has transcriptional inhibitory activity, binds directly to the Tbx5 enhancer, and restricts Tbx5 expression to the prospective forelimb region. It has been reported that the combination of specific Hox genes with different functions is important for determining the posterior end of the forelimb bud region. In one study, overexpression of dominant-negative HoxC9 inhibited the function of the Hox9 paralogs (Hoxa9, b9, c9 and d9), which are expressed in the lateral plate mesoderm (LPM) in the prospective flank region. By combining the overexpression of HoxB4 with the dominant-negative HoxC9, the Tbx5 expression domain expanded posteriorly (Moreau et al., 2019). In this embryo, the position where the forelimb bud formed also shifted to the posterior side. The expression boundary between Hoxb4 and Hoxb9 is correlated with the posterior position of the forelimb bud in avian species (Moreau et al., 2019) (Fig. 1A). This result indicates that Hox genes expressed in the LPM are directly involved in the positioning of limb buds, depending on the combination.
Many studies have reported that the position of the limb bud does not change because of a single knockout in mice or the overexpression of a single Hox gene. However, the results indicate that not all Hox transcription factors work similarly: some function as transcriptional activators, whereas others function as transcriptional repressors (Svingen and Tonissen, 2006). In the future, it will be necessary to clarify which Hox gene combination determines the anterior region of the forelimb bud region. It will also be necessary to analyze the molecular mechanisms controlling Hox gene expression in the LPM to determine the forelimb bud region. The Fgf10 gene is the key. Among the genes necessary for limb initiation, Fgf10 expression is localized in the prospective forelimb bud region. The expression region of Tbx5 is wider than that of Fgf10. In Fgf10 knockout mice, initial budding occurs, but the expression of Fgf8 in the AER disappears; thus, the limb bud does not grow (Sekine et al., 1999). From this result, it is expected that studying how the expression region of Fgf10 is precisely localized to the prospective forelimb bud region by the Tbx5 and Hox genes will help to elucidate the positioning of the forelimb bud.
Positioning of the hindlimb budsThere are few reports on genes related to the determination of the hindlimb bud region in the LPM compared to those in the forelimb bud. As in the forelimb bud, Fgf10 expression is induced in the LPM of the prospective hindlimb bud region before budding begins. Tbx4 is expressed in the prospective hindlimb bud region and determines hindlimb identity (Takeuchi et al., 1999). In Tbx4 knockout mice, Fgf10 expression is initiated normally in the LPM (Naiche and Papaioannou, 2003). Pitx1 induces Tbx4 as an upstream factor and specifies hindlimb identity in cooperation with Tbx4; however, the hindlimb still forms in Pitx1 knockout mice (Szeto et al., 1999). Therefore, Pitx1 and Tbx4 are thought to be involved in determining hindlimb identity. The mechanism by which Fgf10 expression is localized in the prospective hindlimb bud region remains unknown. However, similar to the prospective forelimb bud region, Hox genes may also be involved in determining the prospective hindlimb bud region. The position of the hindlimb bud does not change with the ectopic expression of a single Hox gene in mice, nor does it determine the forelimb bud region. Therefore, to date, there is no evidence that Hox genes expressed in the LPM are directly involved in determining the prospective hindlimb bud region. Hox9 quadruple mutants have a sacral position shifted posteriorly by two vertebrae (McIntyre et al., 2007). Since the hindlimb is connected to the sacral vertebrae via the pelvis, it is believed that Hox9 paralogous gene (PG) is involved in the positioning of the prospective hindlimb bud region. However, Hox genes are also expressed in the somites and presomitic mesoderm (PSM), which is the location of the somites’ progenitor cells. It is still unknown which expression region Hox9PG is involved in in the determination of the hindlimb bud region.
Recently, we have shown that the expression of Gdf11, a secretory factor belonging to the transforming growth factor (TGF)-β superfamily, at the developing posterior axial mesoderm (pAM) forms a sacral-hindlimb unit at the same level, along the anterior–posterior axis of the body (Matsubara et al., 2017). We observed that the GDF11 protein induces the expression of Hox11PG, which is essential for the formation of the sacral bone (Wellik and Capecchi, 2003) in the PSM, and can induce the expression of the Hox9–13 genes around the prospective hindlimb bud region in the LPM (Fig. 2A). Implantation of beads soaked with GDF11 in the LPM successfully induced the collective expression of these Hox genes, resulting in a change in the position of the hindlimb bud anteriorly (Matsubara et al., 2017). This suggests that GDF11 functions to form a hindlimb bud in the affected region by inducing the collective expression of the 5' Hox genes in the prospective hindlimb bud region. The sacral vertebrae and hindlimb originate from different tissues – the PSM and LPM, respectively – and can form independently of each other; normally, though, the sacral vertebrae connect to the hindlimb via the pelvis. It is speculated that a specific combination of Hox genes is involved in determining the prospective hindlimb bud region, and thus the formation of the hindlimb at the sacral vertebrae position. This hypothesis is supported by the observation that extra limbs are formed independently of the vertebrae of the body axis when bead-soaked FGF8 is implanted into the flank region (Ohuchi et al., 1998). However, which particular Hox gene is involved in determining the hindlimb bud region is an important question that needs to be addressed in the future.

Our results explain the morphological diversity of hindlimb positions along the anterior–posterior axis of the body. We showed that the timing of the initiation of Gdf11 expression varies among species, resulting in different numbers of vertebrae from the head to the hindlimbs, i.e., diversity in hindlimb positions in tetrapods (Matsubara et al., 2017). The expression region of the 5' Hox genes in the LPM induced by GDF11 and the location of the prospective hindlimb bud region differ between species. The difference in initiation timing of Gdf11 expression brings about a coordinated shift of the sacral–hindlimb unit along the anterior–posterior axis of the body by collectively inducing the expression of the 5' Hox genes, which is a key point that creates the diversity of hindlimb positions.
Additionally, Gdf11 is involved in trunk-to-tail transitions. The human body is formed from the rostral part and extends caudally, and somites subsequently form from the head to the tail as the body’s axis extends. The trunk-to-tail transition refers to how the pattern of the anterior–posterior axis of the body is formed up to the tail, which is formed at the end of the body axis after the central part, the trunk, is formed. This is a research field that focuses on pattern formation in the posterior part of the body. We found that GDF11 expression in the pAM determines the position of the sacral vertebrae and hindlimbs. During development, when the trunk-to-tail transition occurs, the hindlimbs, genital organs and cloaca, which are formed in the lower body, are also formed around the sacral vertebrae, and the caudal vertebrae are then formed at the posterior side. In Gdf11 knockout mice, the sacral–hindlimb unit shifts posteriorly, together with the posterior shift of the genital organs and cloaca (McPherron et al., 1999). Based on this result, TGF-β signaling, including Gdf11, is a factor in determining the position of the hindlimbs and the timing of the formation of the posterior part of the body, including the trunk-to-tail transition. However, the biological mechanism by which GDF11 integrates the positions of the sacral vertebrae and hindlimbs derived from different tissues to form the entire lower body remains unknown. One possibility is that the transcription factor Islet1 is expressed at an early stage of the posterior hindlimb bud downstream of Gdf11 and controls the timing of the trunk-to-tail transition (Jurberg et al., 2013). Forced expression of Islet1 throughout the lower body using the Cdx2 enhancer, whose activity is maintained in the paraxial mesoderm and LPM through later stages of the tail to the forelimb bud level, including the neuroectoderm, mesoderm and endoderm in the caudal region prior to somite formation (Gaunt et al., 2005), resulted in the formation of a tail behind a short trunk, similar to the results of the overexpression of the constitutively active TGF-β receptor, ALK5, using the Cdx2 enhancer (Jurberg et al., 2013). To date, it remains unclear how endogenous Islet1 is involved in determining the timing of the trunk-to-tail transition, as Islet1 knockout mice die due to abnormal heart formation before the hindlimb bud is formed (Cai et al., 2003). Conditional knockout of the Islet1 gene, using the Hoxb6 promoter-derived Cre line, from the developmental stage prior to the formation of forelimb buds in the LPM resulted in the formation of hindlimb buds, although their subsequent elongation was inhibited (Itou et al., 2012). From these results, it is necessary to elucidate which expression zone of endogenous Islet1, excluding its expression in the LPM, is directly involved in determining the timing of the trunk-to-tail transition. Similarly, a model has been proposed in which Oct4, which has the opposite function of GDF11, is expressed in the epiblast and antagonizes GDF11 function to determine the timing of the trunk-to-tail transition (Aires et al., 2016). Forced expression of Oct4 using the Cdx2 enhancer induced a longer trunk, resulting in an increased number of thoracic and lumbar vertebrae. The Cdx2 enhancer can induce exogenous gene expression in many tissues localized to the posterior part of the body, including the early stage epiblast (Gaunt et al., 2005). An important future research target is to determine which expression zone of endogenous Oct4 regulates trunk length. The tissue in which Gdf11 expression begins is species-dependent. For example, epiblast expression has been observed in mouse embryos (McPherron et al., 1999), whereas Gdf11 is expressed in the pAM derived from epiblasts in chick embryos (Matsubara et al., 2017).
Therefore, the timing at which cell identity along the anterior–posterior axis of the body is determined varies among species.
As mentioned above, we found that GDF11 can induce 5' Hox gene expression in the prospective flank region of the LPM. In this experiment, the expression of Hox9–13PG was induced by the implantation of beads soaked with GDF11 protein in the LPM, concomitantly with an anterior shift of the hindlimb. In Gdf11 knockout mice, the position of the hindlimb and other organs located at the posterior of the body is shifted, including the sacral and genital organs and cloaca. These results imply that when GDF11 is expressed, it acts independently on the tissues surrounding the pAM and induces the expression of Hox9–13PG to form organs located in the posterior side of the body, such as the sacral vertebrae, hindlimbs, genitalia and cloaca. We named the system that generates organs cooperatively by controlling the positional relationship between organs as the anatomical integration system (Matsubara et al., 2017). GDF11 is considered to be a functional factor of this system, regulating the formation position of each organ located in the lower body by inducing the expression of the 5' Hox gene in each organ primordium.
Evolution and the initiation timing of Gdf11 along the rostral–caudal axisThe role of GDF11 in lower body formation also suggests a molecular mechanism that produces developmental constraints in the body plan along the anterior–posterior axis of all vertebrates. Patterns along the anterior–posterior axis of vertebrates, including humans, are formed from the anterior (rostral) side, with no exceptions. The organs located in the upper body, including the head and forelimbs, are formed first in order of development followed by the lower body. This patterning period along the anterior–posterior axis of the body corresponds to the elongation stage of the body axis and is known to be a developmental stage in which the morphology and expressed genes are similar across species in vertebrates. This stage of development is called the phylotypic stage (Sander, 1983). The development of vertebrates is often explained using an hourglass model. The phylotypic stage corresponds to the bottleneck at the center of the hourglass and is similar across species in terms of morphology and gene expression. The phylotypic stage is exhibited by all vertebrates during development and is thought to be a developmental stage that displays strong developmental constraints that cannot be changed in the process of evolution. Since the phylotypic stage is the developmental stage in which the Hox genes are expressed, Duboule (1994) proposed the Hox constraint model in which the phylotypic stage is explained by the Einbahnstrasse (one-way) expression of Hox genes along the anterior–posterior axis of the body. In this model, Hox genes are expressed from Hox1PG located on the 3' side (the centromeric side of the chromosome) to Hox13PG located on the 5' side (the telomeric side of the chromosome) in order from the anterior (rostral) side of the body. This model explains the similarity in gene expression and morphology at the axial elongation stage, and was the first model to explain cross-species similarities in morphology and expressed genes at the phylotypic stage at the molecular level. Since GDF11 induces the expression of the 5' Hox genes (Hox9–13) in the lower body, GDF11 apparently functions by switching the expression of the Hox genes from the 3' Hox gene group to the 5' Hox gene group within the Hox constraint during the axial elongation stage. Briefly, before Gdf11 expression is initiated, the expression of the 3' Hox gene group is induced by unknown upstream genes, following Gdf11 gene expression changes, with the expression of the 5' Hox gene group being induced to form the lower body downstream of GDF11 signaling. The bottleneck during the developmental hourglass model is called the phylotypic point, which is at the center of the phylotypic stage. Gdf11 expression is initiated at the phylotypic point, at which GDF11 changes Hox gene expression from 3' Hox genes to 5' Hox genes during the body axis elongation stage (Fig. 2B). The phylotypic point may be defined as the time of onset of Gdf11 expression in each vertebrate embryo. Therefore, I propose a new model, the Gdf11 constraint model, for the mechanism that produces similarities between morphogenesis and gene expression during the phylotypic stage by Gdf11. The Gdf11 constraint corresponds to the axial elongation stage of the developmental process experienced by all vertebrates. The Gdf11 constraint is a universal trait – the moment in animal development when changes are induced in Hox gene expression, particularly from the 3' Hox gene group to the 5' Hox gene group – and establishes the anterior–posterior axis of the body concomitantly with changes in the developmental system from upper-to-lower body formation.
After the limb bud is formed it grows distally. At this point, the mesenchymal cells of the limb bud are covered with ectoderm. The ectoderm at the tip of the limb bud has a bulging structure called the AER, in which FGF4/8/9 are expressed. In triple mutants lacking these genes within the AER, cell death was induced in the mesenchyme (Mariani et al., 2008). Therefore, FGF signaling in the AER is necessary to maintain the correct number of mesenchymal cells and limb elongation. It has also been clarified that WNT5a is essential for limb bud elongation as a secretory factor expressed in the distal limb bud mesenchyme. In WNT5a knockout mice, normal elongation of limb buds is slightly inhibited at the early stages and cartilage elongation is inhibited at later stages (Yamaguchi et al., 1999). Many genes that are expressed region-specifically and involved in pattern formation along the proximal–distal axis have been reported.
To date, genetic studies have revealed genes such as FGF and Wnt5a, which are essential for limb bud elongation along the proximal–distal axis; however, the limb buds in knockout mice lacking these genes still extend distally (Gao et al., 2011). Thus, there may be a gene that has not been identified and/or an unknown molecular mechanism that controls the behavior of the limb bud cells. Because the tissue of the limb bud is thick and large, it is not possible to observe the behavior of all cells simultaneously, even using time-lapse imaging by confocal microscopy. Time-lapse imaging of limb bud cells using slice culture showed that FGF signaling increased cell mobility and motility, and mesenchymal cells of the limb bud moved toward the location where the WNT5A protein was ectopically applied (Gros et al., 2010). These results suggested that WNT5a is expressed in the mesenchyme on the distal side and acts as a chemoattractant to induce cell migration on the proximal side. In fact, it is highly likely that WNT5A affects the limb bud cells during the early stages of limb bud formation.
However, even if the length of the limb bud exceeded 1 mm, it continued to grow along the proximal–distal axis. This suggests that a mechanism other than WNT5A regulates the distally directed elongation of the limb bud.
When the length of the limb bud is of the order of several millimeters, it is difficult to analyze the behavior of all cells inside the limb bud. Therefore, in a recent study, we developed a method for quantitatively analyzing the relative deformation pattern of mesenchymal cells at the cell population level, i.e., several hundreds of cells, by labeling the cell population of the entire limb bud at multiple points with the fluorescent dyes DiI and DiO (Morishita and Suzuki, 2014). The deformation pattern indicates two changes in the shape and size of the cell population per unit time. Using this method, it is possible to determine the extent to which the cell population at each location inside the limb bud grows and changes shape per unit time. The aim of the analysis is that the deformation of the cell population in a region that is not labeled with the fluorescent dye can be estimated from the data of the measured positions using a statistical method. In this estimation method for the deformation pattern, we presumed that the area between the two measured positions per unit time deformed smoothly and without bias. This estimation was verified because the estimated deformation/position matched the experimentally measured deformation/position.
Therefore, we found that the entire cell population of the limb bud elongated distally by stretching along the proximal–distal axis per unit time (Morishita et al., 2015) (Fig. 3). Previous reports have shown that limb bud elongation along the proximal–distal axis cannot be explained by cell proliferation rate alone (Boehm et al., 2010). Elucidation of the mechanism of anisotropic elongation along the proximal–distal axis at the cell population level could become an important research target in the future for understanding distally directed elongation of the entire limb bud. Furthermore, we found that the inhibition of cell proliferation resulted in the deformation of the distally directed elongation of the mesenchymal tissue of the limb bud (Morishita et al., 2015). This result strongly suggests that the cell population elongates along the proximal–distal axis owing to cell movements other than cell proliferation.

As the limb buds grow, the digit primordium can be seen in the autopod from St. 26 on the chick hindlimb bud. Our fingers are formed along the anterior–posterior axis of the limb bud and have a characteristic shape from the thumb to the little finger. In the hindlimbs of chicken embryos, differences in the shape of each digit are easy to identify. Four digits form on the hindlimb of the chick embryo. First, the digit 4 primordium is formed on the posterior side of the autopod and then digits 3, 2 and 1 are formed in order. Each digit has a different shape, size and number of phalanges. The difference in morphology based on these three criteria is called digit identity. The mechanism through which digit identity is determined along the anterior–posterior axis of the limb bud has long been studied and cited as a good model for pattern formation.
Saunders and Gasseling (1968) conducted an experiment in which the posterior part of the limb bud was transplanted to the anterior side while studying the necrosis observed in the limb bud. They observed that this embryo duplicated digits anteriorly as a mirror image. It has been suggested that there is a substance that characterizes individual digit identity in the ZPA (Saunders and Gasseling, 1968). Wolpert et al. (1969) proposed the ZPA theory. They grafted ZPA on the anterior side while releasing diffused molecules, ensuring that each digit’s prospective primordium cells would receive different concentrations from the ZPA along the anterior–posterior axis. The identity of this factor was unknown; however, Riddle et al. (1993) discovered SHH as a secretory factor expressed in the ZPA. Similar to ZPA transplantation, SHH-expressing cells were transplanted to the anterior side of the limb bud to form duplicated digits in a mirror image. Therefore, it was confirmed that the substance expressed in the ZPA that characterizes each digit’s identity is SHH (Fig. 4A). The longer SHH is present in the anterior side, and/or the higher its concentration, the more posterior digits are formed. Thus, it became clear that two types of functions, concentration and time of exposure to SHH, are essential for determining digit identity (Harfe et al., 2004). However, when the Shh gene was disrupted, only digit 1 in the chick hindlimb was formed. This suggests that Shh is required for the proliferation of the posterior limb bud cells to the prospective digit 2, 3 and 4-forming region. However, double-knockout mice of Shh and Gli3, a transcription factor responsible for the intracellular signal transduction of SHH, show polydactyly as the digits are formed; however, digit identity was lost (Litingtung et al., 2002). This indicates that Shh is necessary for determining the appropriate number of digits and their identities. Based on the ZPA theory, SHH acts in a concentration-dependent manner along the anterior–posterior axis of the limb bud, and the memory of SHH is engraved in the cells that give rise to each digit. Subsequently, each digit primordium is developed with a digit identity based on the direct memory of SHH. Recently, detailed analyses of the timing of the SHH function related to the determination of the digits’ number have been reported (Zhu and Mackem, 2017; Huang and Mackem, 2022). This review focuses on how digit identity is determined downstream of the SHH signals. The expression pattern of Shh is absent when formation of the digit primordium occurs. Thus, it is unlikely that SHH acts directly on the digit primordium. SHH functions as a ligand activating the SHH signaling pathway, which induces the expression of target genes. SHH-dependent gene expression is a memory of SHH. When the SHH signal is activated, the transcription factor GLI3 is activated to induce target gene expression. Chromatin immunoprecipitation (ChIP)-seq studies of GLI3 have identified many target genes (Vokes et al., 2008; Lex et al., 2022). Clarifying the molecular nature of the memory of SHH needs to be addressed in future work.

Genetic studies in mice have shown that digits 4 and 5 are formed by a group of descendant cells that express Shh, whereas digit 3 is formed by a subset of these cells (Harfe et al., 2004). Digit 2 is not composed of Shh descendant cells; instead, its identity is determined by SHH diffusion and function. Therefore, it has been proposed that SHH memory is present in the prospective digits’ primordium. In developmental biology, the phenomenon before cells acquire their shape but are in their differentiation direction is known as specification. Specification means that the tissue’s direction of differentiation is dependent on the situation of the various surrounding cells. Based on the ZPA theory, when the digit primordium first developed, due to the SHH memory it was specified, becoming a particular digit. It is thought that the digit primordium receives positional information reflecting each digit’s identity. However, it has not yet been ascertained whether each digit primordium can develop as an expected particular digit after being isolated and placed under conditions where the surrounding environment is neutral. If we can perform this experiment in the future, we can identify when the cells of the prospective digit primordium become a particular digit.
Determination of digit identity by interdigital tissues in the late stage of limb developmentIn the hindlimb of the chick, the digit primordium becomes visible at St. 26. Dahn and Fallon (2000) bisected the digit 3 primordium along the proximal–distal axis and inserted a foil barrier between the bisected digit 3 primordium. Thus, the bisected anterior digit 3 primordium develops into digit 2, whereas the posterior half develops into digit 2. This indicates that digit identity is not determined when the digit primordium begins to form.
After the limb bud is formed, mesenchymal cells proliferate and the limb bud elongates distally. The region where the digit primordium is formed is called the autopod, and each digit primordium is formed from the posterior to the anterior side of the chick hindlimb (Fig. 4B). The digit primordium is an aggregate of cartilage cells, which appears as a white mass from the proximal side toward the AER. The circumference of the digit primordium is a vascularized mesenchyme and an avascular zone exists between the distal part of the digit primordium and the AER. There is a marginal sinus of blood vessels between the avascular zone and digit primordium. The ray from the proximal side of the digit primordium to the AER is referred to as the digital ray (DR). The DR and digit primordium are anatomically different: the proximal region of the DR, except for the avascular zone and AER, is a digit primordium. The number of DRs is equal to the number of digits; the area between the DRs is called an interdigit. After the DR becomes visible, the interdigit can be distinguished from the DR. The autopod consists of a DR and an interdigit arranged alternately along the anterior–posterior axis. After this stage, the interdigit continued to grow distally with the DR, and apoptosis was subsequently induced at St. 31 in the chick embryo. The apoptotic interdigits disappeared, and individual digits that had each digit’s identity were formed after 10 d of incubation. Dahn and Fallon (2000) found that when an interdigit was removed, its anterior digit identity was transformed into one digit anterior. For example, when interdigit three (ID3) was removed, its anterior digit, digit 3, was transformed into digit 2. Based on this result, it was clarified that the interdigital region, which had been thought to undergo apoptosis and disappear, characterized the identity of the digit located on the anterior side. That is, in the intact state, ID3 is indispensable for determining digit 3 identity by providing positional information to DR3.
Another question is whether the digit primordium that first becomes visible is already specified as a particular digit with positional information due to an SHH memory. In the absence of ID3, it is unlikely that the digit 3 primordium had already been specified as digit 2. The hypothesis was that the digit 3 primordium was determined as digit 2 by the anteriorly located ID2 after ID3 removal. In the definition of specification, it is necessary to check which digit is formed in neutral conditions when the digit 3 primordium is formed, to understand whether it already possesses an SHH memory specifying it as digit 3. From the experiments on interdigital removal and foil barrier insertion, it was expected that the SHH memory would be present in the interdigital region. The next question is, how does the interdigital region determine digit identity? We investigated the location of cells that formed the digit using lineage tracing after the digit primordium was formed. Consequently, cells in the avascular zone located on the distal side of the digit primordium proliferate and are supplied to the vascular zone on the proximal side of the digit primordium, resulting in condensation of the digit primordium’s cartilage (Suzuki et al., 2008). We found that the cells of the digit primordium were derived from a group of cells in a very limited and narrow avascular region. Phosphorylation of SMAD1/5/8, a transcription factor involved in bone morphogenetic protein (BMP) signaling, was observed in the same region where Sox9 is expressed, a marker of precartilage condensation at the tip of the digit primordium. We named this region the phalanx-forming region (PFR) (Suzuki et al., 2008) (Fig. 4C). Because BMPs are expressed in the interdigital region, it was suggested that the BMP signal is transmitted from the interdigital region to the DR via the PFR to determine digit identity. When beads soaked in Noggin, a BMP antagonist, were implanted into ID3, digit 3 was transformed into digit 2, in a similar way to when ID3 was removed (Dahn and Fallon, 2000). Therefore, interdigital BMPs are thought to function in SHH memory. There are some issues that need to be elucidated in the ID model, which is the mechanism for determining digit identity by the interaction between the interdigit and DR via the PFR. Because implantation of beads soaked with Noggin induces digit anteriorization, BMP signals are necessary for determining digit identity. However, there are still no reports that implantation of BMP-soaked beads induces posteriorization of digit identity. These results raise the possibility that factors other than BMP expressed in the interdigit are also involved in determining digit identity. A second question is, how does a DR receive a signal from an interdigit? Because digit 3 identity does not change after the removal of the anteriorly located ID2, it is thought that DRs follow positional information that is posteriorly located. Recently, it was reported that DRs actively receive positional information via the PFR only from posterior IDs (Suzuki and Fallon, 2021). In the future, it will be necessary to determine the molecular mechanism by which PFR cells show a unidirectional response to obtain positional information, specifically from IDs located posterior to their position.
By removing an ID we change its anterior digit identity for a certain period, as a digit’s identity can be changed by ID removal for a specific time period. We have shown that each digit identity is determined from St. 28 to St. 30- for digit 1, St. 27 to St. 30- for digit 2, St. 26- to St. 28+ for digit 3, and St. 25 to St. 26+ for digit 4 in the chick hindlimb (Suzuki et al., 2008). Therefore, the DR receives positional information from a posteriorly located ID for a certain period to determine each digit’s identity. In the subsequent stages, there is no change in digit identity after ID removal. Therefore, this is the stage when digit identity determination is completed.
To further understand digit identity determination, we need to address which tissue the memory of SHH is integrated into and what the molecular entity of the memory of SHH is. In addition, genome-wide analysis combined with next-generation sequencing will be required in the future to elucidate how SHH memory changes depending on the concentration and time of exposure to SHH.
In this review, I discuss the issues to be solved in the next decade by focusing on three targets: limb positioning, proximal–distal limb elongation and digit identity determination. These three issues are similar to each other in terms of the history of research on classic pattern formation mechanisms and of attempts to identify the genes involved. In the future we need to address how micro-behavior at the cellular level forms the shape of the entire limb bud and to identify the molecular entity that passes positional information along the proximal–distal axis in the limb bud and the anterior–posterior axis in the autopod, possibly using single-cell RNA-seq and/or by elucidating region-specific histone modification in a genome-wide manner.
This research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
This work was supported by KAKENHI grant numbers 20H04867 and 22H02673, the Foundation of Kinoshita Memorial Enterprise and the NOVARTIS Foundation (Japan) for the Promotion of Science.