2024 Volume 99 Article ID: 24-00017
2024 Volume 99 Article ID: 24-00017
Intraspecific variation in specialized metabolites plays a crucial role in the adaptive response to diverse environments. Two major subspecies, japonica and indica, are observed in Asian cultivated rice (Oryza sativa L.). Previously, we identified (3R)-β-tyrosine, a novel nonproteinogenic β-amino acid in plants, along with the enzyme tyrosine aminomutase (TAM1), which is required for β-tyrosine biosynthesis, in the japonica cultivar Nipponbare. Notably, TAM1 and β-tyrosine were preferentially distributed in japonica cultivars compared with indica cultivars. Considering its phytotoxicity and antimicrobial activity, intraspecific variation in β-tyrosine may contribute to the defensive potential of japonica rice. Investigation of the evolutionary trajectory of TAM1 and β-tyrosine should enhance our understanding of the evolution of rice defense. However, their distribution patterns in O. rufipogon, the direct ancestor of O. sativa, remain unclear. Therefore, in this study, we extensively examined TAM1 presence/absence and β-tyrosine content in 110 genetically and geographically diverse O. rufipogon accessions and revealed that they are characteristically observed in the ancestral subpopulation of japonica rice, while being absent or slightly accumulated in other subpopulations. Thus, we conclude that TAM1 and β-tyrosine in japonica rice are likely derived from its ancestral subpopulation. Furthermore, the high and low TAM1 possession rates and β-tyrosine content in japonica and indica rice, respectively, could be attributed to distribution patterns of TAM1 and β-tyrosine in their ancestral subpopulations. This study provides fundamental insights into the evolution of rice defense.
In some species, intraspecific variation in specialized metabolites contributes to environmental adaptations. For example, white clover (Trifolium repens) exhibits two population types, one with and the other without cyanogenic glucosides (Kakes, 1989). Because cyanogenic glucosides are toxic to herbivores when metabolized by β-glucosidase (Møller, 2010), the cyanogenic variant is advantageous under herbivore pressure (Kakes, 1989). Conversely, the acyanogenic phenotype thrives in colder climates with reduced herbivore pressure (Hayden and Parker, 2002).
Two major subpopulations, which are often referred to as subspecies japonica and indica, are observed in Oryza sativa L. (Garris et al., 2005). In previous metabolomics analyses of the japonica cultivar Nipponbare and the indica cultivar Kasalath, we identified (3R)-β-tyrosine, a novel nonproteinogenic β-amino acid in plants, exclusively in Nipponbare (Yan et al., 2015). We also identified the gene required for β-tyrosine biosynthesis (tyrosine aminomutase) in Nipponbare, proposing TAM1 as the gene’s name (Yan et al., 2015). TAM1 converts L-tyrosine directly to β-tyrosine by shifting its amino group to the β-position (Yan et al., 2015). Furthermore, in an extensive investigation of TAM1 presence/absence and β-tyrosine content in cultivated rice, they were preferentially observed in japonica cultivars, which are primarily cultivated in temperate environments, compared with indica cultivars, predominantly grown in tropical environments (Yan et al., 2015). Moreover, β-tyrosine is secreted from roots and shows phytotoxicity; it also accumulates in young leaves and has antimicrobial activity (Yan et al., 2015; Sakamoto et al., 2022). Thus, TAM1 and β-tyrosine may contribute to the allelopathic and antibacterial potential of japonica rice. Investigating their evolutionary history should enhance our understanding of the evolution of rice defense. However, their distribution patterns in Oryza rufipogon, the direct ancestor of O. sativa, remain unclear. Therefore, in this study, TAM1 and β-tyrosine were identified in O. rufipogon and then genotypes at TAM1 and β-tyrosine amounts were assessed in 110 genetically and geographically diverse O. rufipogon accessions.
According to Oryzabase (Kurata and Yamazaki, 2006), there are over 650 accessions in O. rufipogon. However, TAM1 and β-tyrosine have not been identified in any accessions. Prior to broader investigation, genomic polymerase chain reaction (PCR) and liquid chromatography–ion trap–time-of-flight-mass spectrometry (LCMS-IT-TOF) analyses of the O. rufipogon accession W1963, which is closely related to japonica rice (Huang et al., 2012), were conducted to confirm the presence of TAM1 and β-tyrosine in the O. rufipogon accession. Given that TAM1 is present in Nipponbare but absent in Kasalath (Yan et al., 2015), these cultivars were used as positive and negative controls, respectively, in PCR analysis. PCR-based genomic DNA amplification yielded a product from W1963 and Nipponbare, but not from Kasalath (Fig. 1A). LCMS-IT-TOF analysis of W1963 seeds revealed a peak with a retention time (9.4 min), m/z (352.12) and fragment pattern (m/z 352.12→171.05) identical to those of the β-tyrosine standard (Fig. 1B, 1C and Supplementary Fig. S1). These results confirm that O. rufipogon, or at least W1963, possesses TAM1 and β-tyrosine; therefore, we conducted a broader investigation into their distribution in diverse O. rufipogon accessions.

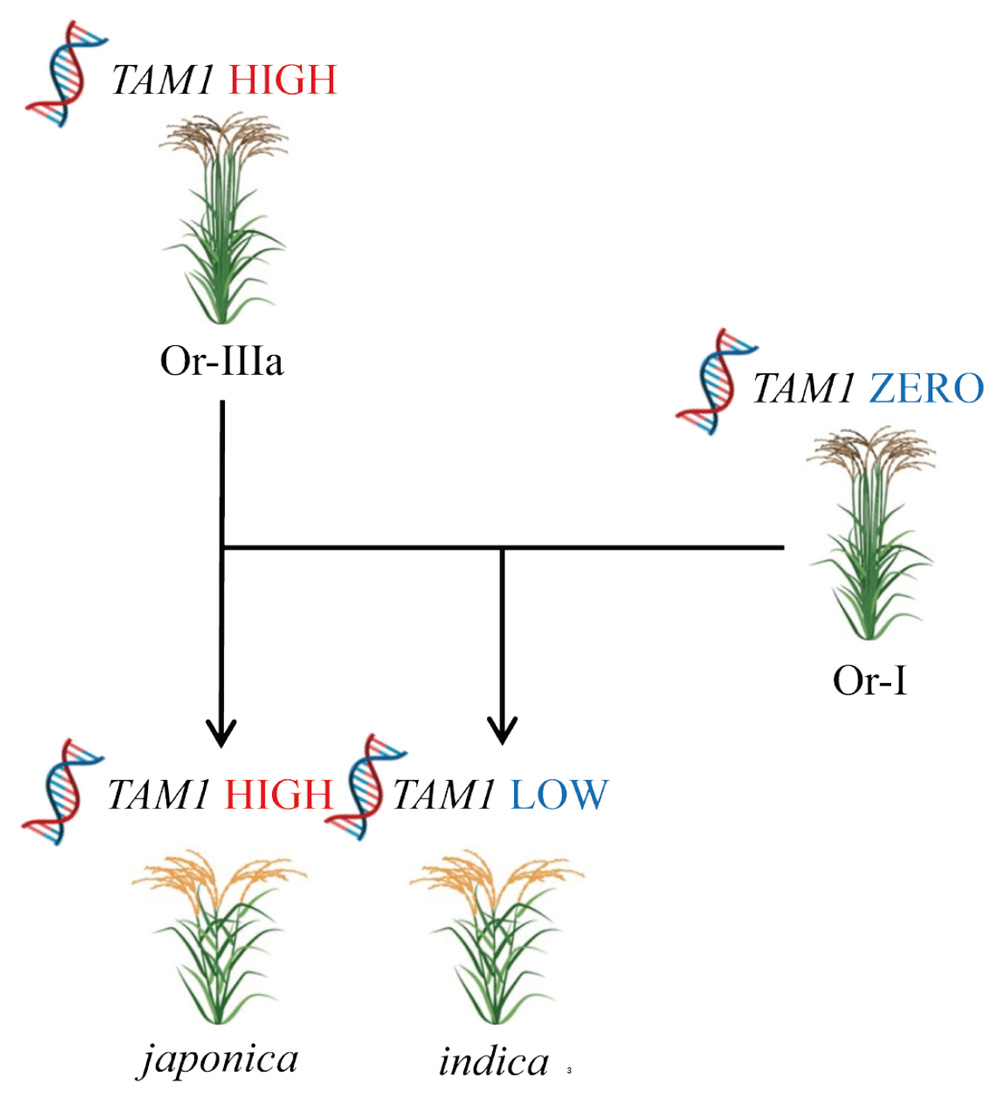
Three subpopulations, Or-I, Or-II and Or-III, have been suggested to have diverged in O. rufipogon based on their population structure. Phylogenetic analysis, using whole genomes and domestication loci, suggested that japonica was first domesticated from Or-III in southern China and later hybridized with Or-I in Southeast Asia and South Asia, giving rise to indica (Huang et al., 2012). This conclusion has been widely accepted in recent years (Kariya et al., 2020; Lee et al., 2022; Shi et al., 2023), despite the controversial origin of cultivated rice (Jing et al., 2023). Therefore, to understand the distribution patterns of TAM1 and β-tyrosine in the ancestral subpopulations of Asian cultivated rice, we focused on Or-I and Or-III. Initially, we investigated TAM1 presence/absence in 110 genetically diverse accessions, comprising 69 Or-I and 41 Or-III accessions, using genomic PCR analysis, following the approach applied to W1963. PCR amplification yielded a single DNA band, which was observed in Nipponbare but not in Kasalath, that was present in six out of 110 O. rufipogon accessions: W1560, W1943, W1945, W1952, W1957 and W1958 (Supplementary Table S1 and Supplementary Fig. S2). These accessions belonged to Or-III, with a TAM1 possession rate of 14.6% (six out of 41 accessions; Fig. 2A). Conversely, no band was observed in any Or-I accession, resulting in a 0% TAM1 possession rate (zero out of 69 accessions; Fig. 2A). These results indicate a significant presence of TAM1 in Or-III (Fisher’s exact test, P < 0.005). Or-III is further divided into two groups based on geographic distribution: Or-IIIa, considered the immediate progenitor of japonica and distributed in China, and Or-IIIb, distributed in other regions (Huang et al., 2012). Investigating TAM1 possession rates between Or-IIIa and Or-IIIb revealed a significantly higher prevalence in Or-IIIa (71.4%; five out of seven accessions) compared with Or-IIIb (2.9%; one out of 34 accessions) (Fisher’s exact test, P < 0.0005; Fig. 2B), indicating TAM1’s characteristic association with Or-IIIa. Thus, we conclude that TAM1 in japonica rice is likely derived from Or-IIIa (Fig. 3). Considering the domestication process of Asian cultivated rice (Huang et al., 2012), the high and low possession rates of TAM1 in japonica and indica cultivars, respectively, may be attributed to the high and low (zero in this study) frequency of TAM1 in Or-IIIa and Or-I (Fig. 2A and 3).

Additionally, fragments from the six accessions were Sanger-sequenced, and their coding sequences were compared to that of Nipponbare (DDBJ accession numbers LC802100, LC802101, LC802102, LC802103, LC802104 and LC802105 for W1560, W1943, W1945, W1952, W1957 and W1958, respectively). This analysis revealed identical nucleotide sequences between TAM1 of W1560, W1943 and W1945 and that of Nipponbare, supporting the notion that japonica rice inherited TAM1 from Or-IIIa. In contrast, we identified four, two and one single-nucleotide polymorphisms (SNPs) in TAM1 of W1952, W1957 and W1958, respectively (Supplementary Fig. S3A). For W1952, these comprised T997C, C1029A, G1035A and C1185T; for W1957, they included C425G and A1204T; and for W1958, the SNP was T997C. Translating nucleotide sequences into amino acid sequences, TAM1 of W1952 and W1958 were found to be identical to that of Nipponbare (Supplementary Fig. S3B), despite the presence of some SNPs in these accessions. In contrast to other accessions, W1957 displayed double amino acid substitutions P142R and T402S (Supplementary Fig. S3C).
To determine whether the TAM1 presence/absence aligned with β-tyrosine levels, and to examine the effect of amino acid substitutions found in TAM1 of W1957 on its function, we conducted liquid chromatography–mass spectrometry (LC/MS) analysis on seeds of the 110 accessions. Nipponbare and Kasalath served as positive and negative controls, respectively. LC/MS results revealed that all analyzed accessions, including the negative control Kasalath, possessed β-tyrosine, albeit in very low concentrations in O. rufipogon accessions lacking TAM1 and Kasalath (Fig. 4). The detection limit for β-tyrosine with our analytical system was 14.4 pg/mg fresh weight. Importantly, β-tyrosine was not detected in the extraction solution after all sample processing procedures as shown in ‘control’ in Fig. 4, confirming that its presence in O. rufipogon accessions without TAM1 and Kasalath was not due to contamination. Dunnett’s test was used to compare β-tyrosine concentrations between the 110 accessions and Kasalath control, revealing that seven accessions (W2265, W1560, W1943, W1945, W1952, W1957 and W1958) and the positive control Nipponbare exhibited significantly higher β-tyrosine concentrations relative to Kasalath (Fig. 4). Therefore, all accessions possessing TAM1 (W1560, W1943, W1945, W1952, W1957 and W1958; Supplementary Table S1) produce substantial amounts of β-tyrosine, whereas those lacking TAM1, except for W2265, produce only minimal amounts (Fig. 4). Given that W1943, W1945, W1952, W1957 and W1958 belong to Or-IIIa, it is evident that Or-IIIa acquired the ability to produce a substantial amount of β-tyrosine. Thus, we conclude that β-tyrosine is also preferentially distributed in Or-IIIa. Despite amino acid substitutions in its TAM1, W1957 accumulated a larger amount of β-tyrosine than positive control Nipponbare, implying no negative effect of P142R and T402S on TAM1’s function.
Notably, W2265 exhibited a substantial amount of β-tyrosine, despite PCR amplification not resulting in any products from this accession (Fig. 4 and Supplementary Fig. S2). According to PCR analysis results, W2265 likely lacks TAM1. However, the primers used in PCR analysis may not have been suitable for amplifying TAM1 in W2265. Despite testing a different primer set, we did not successfully amplify a fragment from W2265 under conditions where PCR amplification yielded a single DNA band from the positive control Nipponbare but not from the negative control Kasalath (Supplementary Fig. S4). These findings support the notion that W2265 produces a significant amount of β-tyrosine without relying on TAM1. Given that all 110 accessions accumulated at least small amounts of β-tyrosine regardless of TAM1 possession (Fig. 4), some enzyme other than TAM1 likely holds the potential to biosynthesize β-tyrosine, and exhibits high activity in W2265. TAM1 shares a similar amino acid sequence with phenylalanine ammonia lyase (PAL), which is responsible for transforming L-phenylalanine into trans-cinnamic acid (Yan et al., 2015). Among eight PALs in rice (OsPAL1–8), OsPAL8 shows 95% overall identity at the amino acid sequence level with TAM1 (Yan et al., 2015). Additionally, TAM1 and PAL share the same catalytic moiety, 3,5-dihydro-5-methylidene-4H-imidazole-4-one (MIO), indicating their similar catalytic mechanism (Barros and Dixon, 2020). In the monocot grass family, PAL uses L-tyrosine as a substrate in addition to L-phenylalanine, producing coumarate (Barros and Dixon, 2020), suggesting that PAL in rice can capture L-tyrosine. Therefore, it is plausible that PAL generates a small amount of β-tyrosine as a byproduct in all accessions, and the side reaction is highly active in W2265. However, further studies are necessary to identify the enzyme(s) required for β-tyrosine biosynthesis in W2265. Considering that β-tyrosine biosynthesized by TAM1 is the (R)-enantiomer (Yan et al., 2015; Walter et al., 2016), identification of the stereochemistry of β-tyrosine in W2265 and other accessions without TAM1 may provide additional information on the property of enzyme(s) of interest.

Having investigated the distribution patterns of TAM1 and β-tyrosine in O. rufipogon, we revealed their predominant distribution in Or-IIIa. We conclude that these traits were likely inherited by japonica cultivars through domestication. Furthermore, the high and low TAM1 possession rates and β-tyrosine content in japonica and indica rice, respectively, are probably due to the distribution patterns of TAM1 and β-tyrosine in their ancestral subpopulations, Or-IIIa and Or-I. Our results imply potential advantages of possessing TAM1 and β-tyrosine in southern China and temperate environments against some environmental pressures such as weeds and pathogens.
The wild rice accessions used in this study were distributed from the National Institute of Genetics supported by the National Bioresource Project, MEXT, Japan. This research was supported by a Grant-in-Aid for JSPS Fellows (22J23407).