Abstract
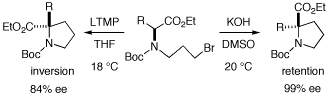
We have developed asymmetric induction based on memory of chirality. This enables highly enantioselective synthesis of unusual amino acids with a tetra-substituted carbon center, new cyclic amino acids, and multi-substituted nitrogen heterocycles. These reactions proceed via enolate intermediates with dynamic axial chirality. Due to dynamic nature of the chiral enolate intermediates, racemization of them gradually takes place. A typical chiral enolate intermediate undergoes racemization with a barrier of 16.0 kcal/mol and the corresponding half-life of 22 h at -78 °C . The chiral enolate is expected to undergo racemization very quickly at 20 °C with a half life of 0.05 sec. These backgrounds prompt us to avoid asymmetric reactions at room temperature. However, we report here that several reactions proceed in up to 99% ee based on memory of chirality even at room temperature.