Abstract
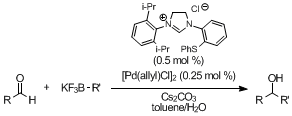
Because of the usefulness of organoboronic acids such as low toxicity and easy manipulation and the importance of the addition products as intermediates, transition metal-catalyzed 1,2-addition reactions of organoboronic acids to aldehydes have been attracting much attention. Recently, potassium organotrifluoroborates have been focused as alternatives to organoboronic acids because of their superior features. In spite of the advantages, only two types of rhodium(I)-phosphine complexes as catalysts for the transition metal-catalyzed 1,2-addition of potassium organotrifluoroborates to aldehydes were reported. We achieved the palladium-catalyzed 1,2-additon reactions of potassium aryl- and alkenyltrifluoroborates to aldehydes using the thioether-imidazolinium carbene ligand in good to excellent yields. They were carried out rapidly using 0.5 mol % of catalyst loading with good substrate tolerance, giving a variety of carbinol derivatives.