2015 Volume 84 Issue 1 Pages 30-36
2015 Volume 84 Issue 1 Pages 30-36
To obtain basic information about doubled haploid plants in Citrus, in the present study, we investigated the morphological characteristics in doubled haploid induced by colchicine-treated axillary shoot buds of a haploid plant from ‘Banpeiyu’ pummelo [C. maxima (Burm.) Merr.]. We also evaluated the reproductive potential of the doubled haploid as a male or a female parent by crossing with some diploids. In term of the results, this doubled haploid had significantly large leaf, flower and fruit compared with those of the original haploid plant. Moreover, the doubled haploid showed higher pollen fertility (84.1% stainability and 32.9% pollen germination rate) and a larger number of seeds (47.2 developed seeds per open-pollinated fruit) than the haploid. In the reciprocal crosses between the doubled haploid and some diploids, many developed seeds were obtained. These seeds germinated normally and developed into diploid seedlings. These results show that the doubled haploid will be valuable for genetic analysis and possibly for planned breeding.
Haploid and doubled haploid plants are of great value for genetic analysis and premeditated breeding (Bajaj, 1990; Germanà, 2006; Ochatt and Zhang, 1996). This is especially the case for woody species, which are generally characterized by a long reproductive cycle, a high degree of heterozygosity, a large plant size, and self-incompatibility.
In fruit crops, therefore, haploids have been obtained from some species such as kiwifruit [Actinidia deliciosa (A. Chev.) C. F. Liang & A. R. Ferguson], apple (Malus pumila Mill.), banana (Musa acuminata Colla), sweet cherry [Prunus avium (L.) L.], peach [P. persica (L.) Batsch], and Japanese pear [Pyrus pyrifolia (Burm. f.) Nakai], and doubled haploids have also been induced by treating shoots and leaves of the haploids with antimitotic agents such as colchicine and oryzalin (Assani et al., 2003; Bouvier et al., 2002; Chalak and Legave, 1996; Hesse, 1971; Höfer and Grafe, 2003; Ochatt and Zhang, 1996; Zhang and Lespinasse, 1991) and spontaneous chromosome doubling of androgenetic microspores in anther culture (Höfer et al., 2008; Okada et al., 2009; Vanwynsberghe et al., 2005).
In Citrus and related genera, some haploid plants have been produced by various techniques such as anther culture (Germanà and Chiancone, 2003; Germanà et al., 1994; Hidaka et al., 1979), interploid hybridization (Germanà and Chiancone, 2001; Oiyama and Kobayashi, 1993; Toolapong et al., 1996), and pollination of irradiated pollen (Aleza et al., 2009; Froelicher et al., 2007; Yahata et al., 2010). However, doubled haploid plants have only been induced by anther culture in Clementine mandarin (C. clementina hort. ex Tanaka) (Germanà and Chiancone, 2003) and ‘Valencia’ sweet orange [C. sinensis (L.) Osbeck] (Cao et al., 2011), and by pollination of irradiated pollen in ‘Clemenules’ Clementine (Aleza et al., 2009). Moreover, the morphological characteristics and the reproductive potential of the doubled haploid in Citrus and related species have not yet been reported.
Toolapong et al. (1996) selected haploid progeny among small seed-derived seedlings obtained from the cross between ‘Banpeiyu’ pummelo and ‘Ruby Red’ grapefruit (C. paradisi Macfad.), and Yahata et al. (2005a) confirmed that this haploid was derived from the female gamete of ‘Banpeiyu’ pummelo. After grafting onto the trifoliate orange [Poncirus trifoliata (L.) Raf.], it showed vigorous growth and flowered for the first time seven years after germination. However, it was difficult to use this haploid for genetic analysis and for planned breeding because it had no fertile female gamete and only a few fertile pollen grains (Yahata et al., 2005a, c, 2011). Therefore, Yahata et al. (2005b) produced the doubled haploid plant of this haploid pummelo using colchicine-treated axillary shoot buds. This doubled haploid showed vigorous growth compared with the original haploid pummelo (Fig. 1A) and fortunately produced many flowers and fruit for the first time 5 years after top-graft onto trifoliate orange (Fig. 1B, C).

The doubled haploid induced by colchicine-treated axillary shoot buds of a haploid plant from ‘Banpeiyu’ pummelo. A: 10-year-old doubled haploid tree (Bar = 30 cm). B: Flowers of the doubled haploid (Bar = 3 cm). C: Fruit of the doubled haploid (Bar = 10 cm).
In the present study, we investigated the morphological characteristics of the doubled haploid induced by colchicine-treated axillary shoot buds of a haploid plant from ‘Banpeiyu’ pummelo. Furthermore, we evaluated the reproductive potential of the doubled haploid as a male or a female parent by crossing with diploid citrus cultivars.
The haploid pummelo obtained from the cross between ‘Banpeiyu’ pummelo and ‘Ruby Red’ grapefruit (Toolapong et al., 1996), the doubled haploid induced by colchicine-treated axillary shoot buds of the haploid plant (Yahata et al., 2005b) and ‘Banpeiyu’ pummelo were used in the present study. These plant materials were grafted onto trifoliate orange, and maintained for approximately 10 years in the greenhouse of the Faculty of Agriculture, Shizuoka University, before being used for the experiment.
The morphological characteristics of fully expanded leaves (i.e., leaf blade size, leaf weight per unit, guard cell size, and stoma density) and flowers just before bloom (i.e., sizes of flower bud, petal, pistil, ovary, and pollen, and numbers of petals, stamens, and ovules per ovary) were measured using 20 samples. The characteristics of open-pollinated fruit investigated were size, numbers of locules and seeds, soluble solid content (SSC), and titratable acidity (TA). For each measurement, 20 samples were used in the doubled haploid, 5 samples in the haploid, and 10 samples in ‘Banpeiyu’ pummelo.
Pollen fertility was evaluated by stainability and in vitro germination. Pollen stainability was estimated by staining the samples with 1% acetocarmine after squashing nearly mature anthers on a slide glass. In vitro germination of the pollen grains was performed on microscope slides covered with a 2-mm layer of 1% (w/v) agar medium containing 10% sucrose. Five stamens, each from a different flower, were rubbed on the agar medium, and the slides were then incubated for 10 h in a moistened chamber at 25°C in the dark. Each test evaluated 1000 grains with five repetitions.
Crossing for evaluation of the reproductive potential of the doubled haploidThe doubled haploid, the haploid pummelo, and 6 diploid species and cultivars of Citrus [‘Banpeiyu’ pummelo, ‘Kiyomi’ tangor (C. unshiu Marcow. × C. sinensis Osbeck), ‘Miyauchi-Iyokan’ (C. iyo hort. ex Tanaka), Koji (C. leiocarpa hort. ex Tanaka), Hyuga-natsu (C. tamurana hort. ex Tanaka), and Hassaku (C. hassaku hort. ex Tanaka)] were used for reciprocal crosses.
The flowers were pollinated immediately after emasculation and covered with paraffin paper bags. Seeds were collected from each fruit of the crosses at maturity. The seeds were extracted from each fruit and classified into two groups, namely, developed (normal development) and undeveloped (empty) seeds. The seeds were then placed on moistened filter paper and maintained at 25°C. After germination, the seedlings were transplanted into pots containing vermiculite and transferred to a greenhouse.
Ploidy level analysisA total of 30–50 seedlings per cross combination were measured by flow cytometry, except for the cross between ‘Miyauchi-Iyokan’ and the haploid. Young leaves were collected from their seedlings, chopped with a razor blade in 2 mL of buffer solution containing 1.0% (v/v) Triton X-100, 140 mM mercaptoethanol, 50 mM Na2SO3 and 50 mM Tris-HCl at pH 7.5, and incubated for 5 min according to the preparation method of Yahata et al. (2005a). Crude samples were filtered through Miracloth (Merck KGaA, Darmstadt, Germany) and stained with 25 μg·L−1 propidium iodide (PI). The relative fluorescence of total DNA was measured for each nucleus with a Flow Cytometry System (EPICS XL; Beckman Coulter, Fullerton, CA, USA) equipped with an argon laser (488 nm, 15 mW).
After flow cytometry analysis, chromosome observation was performed in 3 seedlings obtained from each cross combination. Root tips (approximately 5 mm long) were excised from their seedlings, immersed in 2 mM 8-hydroxyquinoline for 24 h at 10°C, and fixed in a mixed solution of ethanol and acetic acid (3:1) for 24 h at 10°C. Enzymatic maceration and air-drying were performed according to the method of Fukui (1996) with some modifications. Root tips were washed in distilled water to remove the fixative and then macerated in an enzyme mixture containing 2% (w/v) Cellulase Onozuka RS (Yakult Pharmaceutical Ind. Co. Ltd., Tokyo, Japan), 1% (w/v) Macerozyme R-200 (Yakult Pharmaceutical Ind. Co. Ltd.), 0.3% (w/v) Pectolyase (Kyowa Chemical Products Co. Ltd., Osaka, Japan), and 200 mM EDTA at 37°C for 20 min. The macerated samples were rinsed with distilled water and a fixative solution was added. The mixtures were transferred to glass slides. After the slides had been air-dried, the chromosomes were stained with 2% Giemsa solution (Merck KGaA) in 1/30 M phosphate buffer (pH 6.8) for 30 min, rinsed with distilled water, air-dried and observed under an optical microscope (BX51; Olympus, Tokyo, Japan).
The morphological characteristics of the doubled haploid were compared with those of the haploid and ‘Banpeiyu’ pummelo. The sizes of leaf and guard cell of the doubled haploid were larger than those of the haploid and almost equal to those of ‘Banpeiyu’ pummelo (Table 1; Fig. 2A). Flower organ of the doubled haploid showed normal morphology (Fig. 2B). The flower and pollen of the doubled haploid were also significantly larger than those of the haploid, and no difference in flower and pollen size was observed in comparison to those of ‘Banpeiyu’ pummelo (Tables 2 and 3). On the other hand, the doubled haploid had a significantly reduced number of ovules per ovary, that is, approximately half, compared with that of ‘Banpeiyu’ pummelo. In addition, the pollen fertility of the doubled haploid (84.1% stainability and 32.9% pollen germination rate) was remarkably higher than that of the haploid (2.3% and 0.4%) (Fig. 3), but it was significantly lower than that of ‘Banpeiyu’ pummelo (97.7% and 88.2%). The fruit weight of the doubled haploid was approximately 900 g, which was approximately half that of ‘Banpeiyu’ pummelo (Table 4; Fig. 2C). The doubled haploid produced a small number of locules compared with the ‘Banpeiyu’ pummelo. Furthermore, the number of seeds per fruit obtained from ‘Banpeiyu’ pummelo was approximately 100, whereas that of the doubled haploid was significantly less, approximately 60. The average size of the normal seeds from the doubled haploid was smaller than that of the seeds from ‘Banpeiyu’ pummelo. On the other hand, there were no significant differences in both SSC and TA of fruit juice among the haploid, ‘Banpeiyu’ pummelo, and the doubled haploid.

Comparison of morphological characteristics of leaf in ‘Banpeiyu’ pummelo, the haploid and the doubled haploid.

The morphological characteristics of leaves (A, Bar = 5 cm), flowers (B, Bar = 3 cm), and fruit (C, Bar = 10 cm) in ‘Banpeiyu’ pummelo (left), the haploid (center), and the doubled haploid (right).

Comparison of morphological characteristics of flower in ‘Banpeiyu’ pummelo, the haploid and the doubled haploid.

Comparison of morphological characteristics of pollen grain in ‘Banpeiyu’ pummelo, the haploid and the doubled haploid.

Stainability by 1% acetocarmine (A, Bar = 50 μm) and in vitro germination (B, Bar = 50 μm) in pollen of the doubled haploid.

Comparison of morphological characteristics of fruit in ‘Banpeiyu’ pummelo, the haploid and the doubled haploid.
Haploids of fruit crops generally show poor growth and their leaves, flowers, and fruit tend to be smaller than those of diploid plants (Aleza et al., 2009; Chalak and Legave, 1996; Dweikat and Lyrene, 1990; Höfer and Lespinasse, 1996; Pooler and Scorza, 1995; Toyama, 1974; Zhang and Lespinasse, 1991). On the other hand, information on the morphology of doubled haploids has rarely been reported for fruit crops, although doubled haploids have been produced in several species, for example, kiwifruit, apple, banana, sweet cherry, peach, and Japanese pear (Germanà, 2006). Recently, some doubled haploids of apple were produced by in vitro androgenesis and in situ parthenogenesis, and their morphology and reproductive potential have been reported (Höfer et al., 2008; Okada et al., 2009; Vanwynsberghe et al., 2005), which showed that most of the doubled haploid apple lines had smaller leaves, flowers, and fruit than the original diploid cultivars, and some of these doubled haploid lines also showed aberrant morphology of flowers (Höfer et al., 2008; Okada et al., 2009; Vanwynsberghe et al., 2005). In the present study, on the other hand, doubled haploid pummelo showed normal flower morphology, whereas the haploid showed abnormalities such as the adhesion of pistils and stamens in the flowers (Yahata et al., 2005a). Furthermore, this doubled haploid had significantly large leaf, flower, and fruit compared with those of the original haploid plant, and showed similar morphology to that of ‘Banpeiyu’ pummelo.
Some doubled haploid lines of apple showed low or no pollen fertility and reduction of seed number compared with that of original diploid cultivars (Okada et al., 2009; Vanwynsberghe et al., 2005). The doubled haploid pummelo in the present study also showed similar phenomena, although not severer than those of doubled haploids of apple; namely, the doubled haploid showed lower pollen fertility and a smaller number of seeds than ‘Banpeiyu’ pummelo. It was considered that the reduction of the number of seeds was due to a decrease in the number of ovules per ovary. On the other hand, the cause of the decrease in pollen fertility is not clear. To account for the depression of pollen fertility in this doubled haploid pummelo, detailed cytological observation, especially at meiosis, remains necessary. Furthermore, because the variations of morphological characteristics and reproductive functions have been observed among autotetraploid induced by colchicine treatment (Nukaya et al., 2011; Rêgo et al., 2011; Wu et al., 2012), and given the influence of homogenization, it seems that further investigation of their aberrations in the doubled haploid is required. Additionally, because only one doubled haploid line was investigated in the present study, it will be necessary to produce a lot of doubled haploids, for investigation of their general reproductive functions in the future.
In order to evaluate the reproductive potential of the doubled haploid, crosses with the haploid and some diploid cultivars were carried out in the present study (Table 5). In the reciprocal crosses between the haploid and diploid cultivars, the haploid showed no fruit set as a seed parent, and low fruit set and a few developed seeds as a pollen parent. On the other hand, when the doubled haploid was used as the seed and/or pollen parent, a lot of fruit and developed seeds were obtained in the reciprocal crosses between the doubled haploid and diploid cultivars. These developed seeds germinated almost normally (Fig. 4A), and all of the seedlings had large wing leaves, which is a typical feature of the doubled haploid. FCM analysis of all those seedlings showed that their fluorescence intensity coincided with that of the diploid control. The chromosome count of root tips revealed that all of the seedlings examined had 18 chromosomes (Fig. 4B).
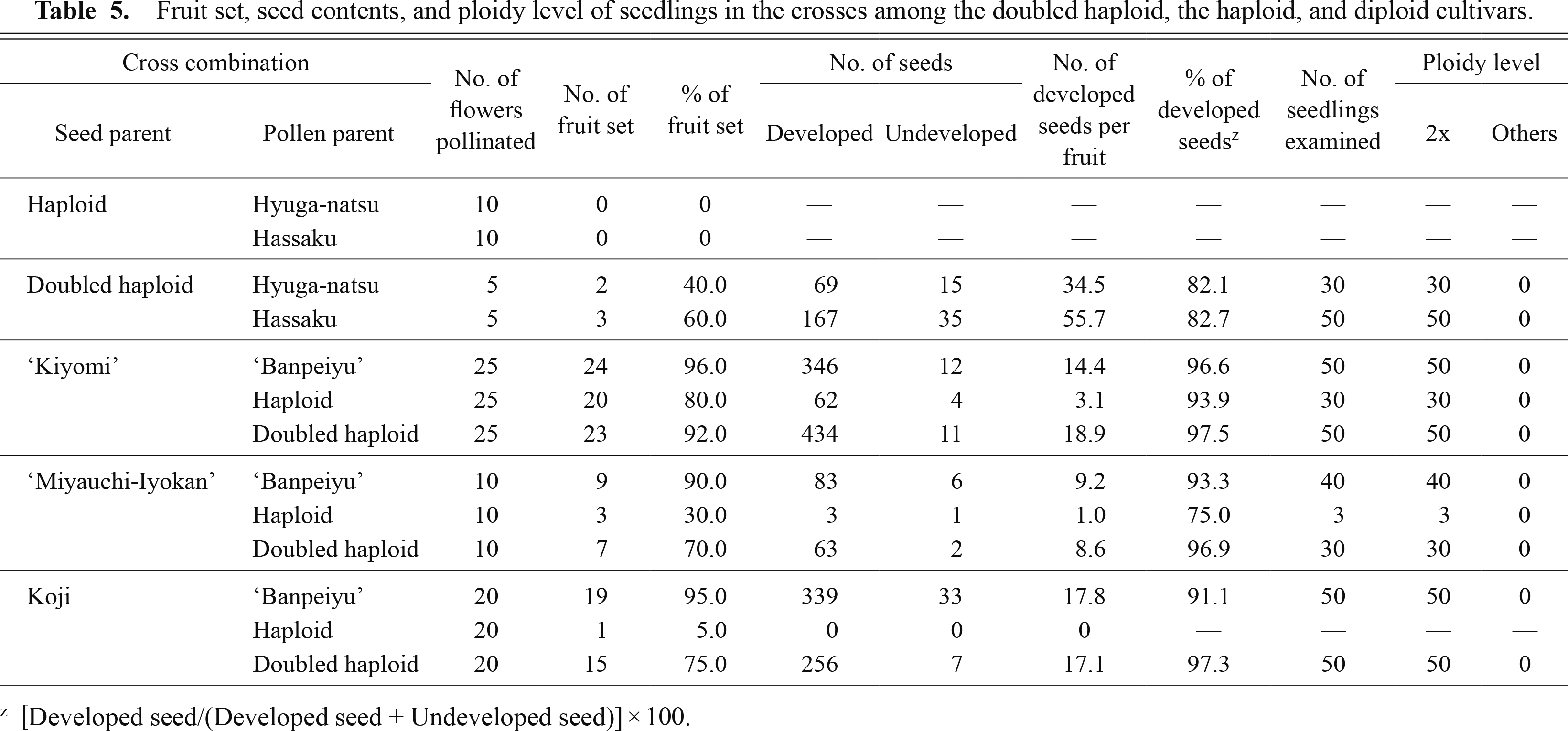
Fruit set, seed contents, and ploidy level of seedlings in the crosses among the doubled haploid, the haploid, and diploid cultivars.

The seedlings obtained from the reciprocal crosses between the doubled haploid and some diploid cultivars (A, Bar = 3 cm) and the metaphase chromosomes in a root tip cell in one of the seedlings (B, 2n = 2x = 18, Bar = 10 μm).
In apple, it was difficult to use the doubled haploid lines as breeding materials because most of them had low and/or no reproductive potential, and no or only a few progeny were obtained in their cross combinations (Okada et al., 2009; Vanwynsberghe et al., 2005). In the present study, on the other hand, when the doubled haploid was used as both seed and pollen parents, a lot of developed seeds and diploid progeny were obtained. This result showed that the doubled haploid pummelo has no problem in term of the reproductive potential of female and male gametes.
In conclusion, the doubled haploid induced using colchicine-treated axillary shoot buds of a haploid plant from ‘Banpeiyu’ pummelo showed similar morphology to that of ‘Banpeiyu’ pummelo, and had significantly large leaf, flower and fruit compared with those of the original haploid plant. The doubled haploid also showed higher pollen fertility and a larger number of seeds than the haploid. In the reciprocal crosses with some diploid cultivars, the doubled haploid produced many developed seeds as both seed and pollen parents. These seeds germinated normally and developed into diploid plants. In Citrus, recently, a rough draft of the genome was completed using the haploid Clementine mandarin and the doubled haploid sweet orange (Ollitrault et al., 2012; Xu et al., 2013). This genomic information has also been applied for the development of DNA markers, genetic analysis, and the production of new cultivars (Ahmad et al., 2013; Cuenca et al., 2013; Garcia-Lor et al., 2013). Our doubled haploid pummelo will also be utilized as alternative material for these genetic and breeding studies.
The authors are grateful to Dr. Masahiro Mii of the Faculty of Horticulture, Chiba University, for his advice and critical reading of this manuscript. The authors also thank Mr. Kiyoto Harusaki for kindly providing the experimental materials.