2015 Volume 84 Issue 2 Pages 111-121
2015 Volume 84 Issue 2 Pages 111-121
To investigate the mechanism of yield increase by elevated carbon dioxide (CO2) and fogging in Japanese tomato cultivars (Solanum lycopersicum), total above-ground dry matter (TDM), fraction of dry matter distribution to fruit (FDF), and photosynthetic characteristics were measured in 3 Japanese cultivars grown in elevated CO2 with fogging and ambient CO2 without fogging. Fresh fruit yield and TDM were improved by the elevated CO2 and fogging in the 3 Japanese cultivars. Light use efficiency (LUE) was also increased by the elevated CO2 and fogging. No significant decrease in FDF was observed by the elevated CO2 and fogging in 2 Japanese cultivars, ‘Asabiyori 10’ and ‘Junkei Aichi Fast’. Thus, the increase in TDM by higher LUE contributed directly to the yield increase in these 2 cultivars. However, FDF in ‘Momotaro York’ was decreased significantly by the elevated CO2 and fogging. Thereby, the yield increase by the elevated CO2 and fogging was diminished in ‘Momotaro York’ in spite of the increase in TDM. The number of trusses having immature fruit in ‘Momotaro York’ under elevated CO2 and fogging was significantly higher than those of the others, although no increase in the number of trusses having immature fruit was observed in the other 2 cultivars. Although vegetative growth characteristics such as leaf area, LAI, and fresh and dry weights of leaves and stem were increased by the elevated CO2 and fogging, no negative effects such as a change in light-extinction coefficient and a decrease in maximum photosynthetic rate were observed. The elevated CO2 and fogging increased the number of harvested fruit but decreased weight per fruit, namely, fruit size, in the 3 cultivars.
The yield of greenhouse tomatoes (Solanum lycopersicum) in The Netherlands has doubled from ca. 30 kg·m−2 per year in 1983 to ca. 60 kg·m−2 per year in 2005 (Kwantitatieve Informatie voor de Glastuinbouw, 2005). The yield of processed tomatoes in California, USA, has also more than doubled over the past 80 years (Barrios-Masias et al., 2014). However, the yield of greenhouse tomatoes in Japan has only slightly increased over the past 30 years (Ministry of Agriculture, Forestry and Fisheries, 2007, http://www.maff.go.jp/j/tokei/kouhyou/sakumotu/sakkyou_yasai/#l). The increase in yield over the past 50 years in Dutch tomato cultivars resulted from the increase in total above-ground dry matter (TDM) production, which resulted from high light use efficiency (LUE) (Higashide and Heuvelink, 2009). In contrast, high-yielding cultivars have not been developed in Japan (Higashide et al., 2012). Japanese tomato breeders have improved fruit quality such as soluble solids rather than yield. The soluble solid content per dry matter content has increased in ‘Momotaro’ (released in 1985) and further in ‘Momotaro Colt’ (released in 2003) compared with those in several of the old cultivars.
The use of high-yielding cultivars is described as most effective to improve the yield of Japanese tomatoes. However, since Japanese consumers and retailers have insisted on a high quality of fruit taste more strongly than on yield, they would reject the introduction of Dutch high-yielding cultivars. Although Japanese breeders have started to develop high-yielding Japanese cultivars, a cultivar that provides both high yield and fruit quality has still not been released. Now, we must attempt to improve the yield by using current Japanese cultivars. To determine causes of the low yield of Japanese tomatoes, researchers compared physiological traits such as photosynthetic rate and dry matter production between Dutch and Japanese cultivars (Higashide and Heuvelink, 2009; Matsuda et al., 2011, 2013; Saito et al., 2011). To improve the yield of Japanese cultivars, Higashide et al. (2014) reported that the yield of ‘Momotaro York’ was increased by grafting onto a Dutch rootstock, ‘Maxifort’, and that this increase resulted from the improvement of LUE.
Elevation of carbon dioxide (CO2) concentration in a greenhouse was also described as effective to improve the yield of greenhouse crops (de Gelder et al., 2005; Fierro et al., 1994; Hicklenton and Jolliffe, 1978; Nederhoff, 1994; Tremblay and Gosselin, 1998; Tripp et al., 1991). CO2 application had been unpopular in Japan since most greenhouse crops such as tomatoes were produced in simple plastic greenhouses without an environmental control system. Recently, many large-scale greenhouses have been built and installed with CO2 application systems in Japan. Effects and efficiencies of CO2 application in those greenhouses have been reported (Kuroyanagi et al., 2014; Takahashi et al., 2012). Moreover, those large greenhouses may also have a fogging system for humidification and cooling by vaporization heat. Humidity in a greenhouse affects the growth and yield of crops such as tomatoes (Bakker, 1991; Jolliet and Bailey, 1992; Jolliet et al., 1993). Consequently, modern greenhouses use both CO2 application and humidification by the fogging system (de Gelder et al., 2012; Harel et al., 2014). Yasuba et al. (2011) investigated temperature, CO2 concentration, and vapor deficit in a greenhouse with CO2 application and fogging, and reported the yield improvement of Japanese tomato cultivars under the environmental conditions. In the present study, we confirmed the effects of CO2 elevation and fogging on Japanese tomato cultivars, and investigated the growth and photosynthetic characteristics, and yield components. We also investigated whether elevated CO2 and fogging improved vegetative growth rather than yield of some Japanese cultivars, and whether the increase in vegetative growth had negative influences on light interception by plants or the photosynthetic rate. To clarify those characteristics, we compared 3 Japanese cultivars between elevated CO2 with fogging and ambient CO2 without fogging.
Three tomato cultivars (Solanum lycopersicum) grown at elevated CO2 with fogging or at ambient CO2 without fogging were compared. We sowed seeds of ‘Asabiyori 10’ (Ab; Asahi Industry, Tokyo, Japan), ‘Junkei Aichi Fast’ (Af; Matsunaga Seeds, Aichi, Japan), and ‘Momotaro York’ (My; Takii Seeds Co., Ltd., Kyoto, Japan) in seed trays filled with nursery soil on 1 November, 2010. My is one of the most popular cultivars in Japan. Previous reports (Higashide et al., 2012; Yasuba et al., 2011) showed that fruit yield in Ab and dry matter allocation to fruit in Af were higher than those in the other Japanese cultivars. The seed trays were placed in a seedling growth chamber (Seedling Terrace; Mitsubishi Plastics Agri Dream, Tokyo, Japan). The seedlings were fertilized every 2 days from below using commercial nutrient solution (High-Tempo; Sumitomo Chemicals, Tokyo, Japan); the solution consisted of 10.7 mM NO3−, 6.3 mM K+, 5.4 mM Ca2+, 1.9 mM Mg2+, 2.4 mM H2PO4−, 3.8 mg·L−1 Fe, 0.38 mg·L−1 Mn, 0.26 mg·L−1 B, 0.15 mg·L−1 Zn, 0.05 mg·L−1 Cu, and 0.07 mg·L−1 Mo, adjusted to electrical conductivity of 1.8 dS·m−1. The seedlings were illuminated with fluorescent lamps using a 16-h day length and a photosynthetic photon flux density (PPFD) of 397 ± 39 μmol·m−2·s−1, and were grown at 900 μmol·mol−1 CO2, and air temperatures of 23 and 17°C (day and night).
On 26 November, 2010, the seedlings were transplanted into 5 or 4 rows of rockwool systems in a greenhouse compartment (13.2 × 12 m) at ambient CO2 without fogging (AMC), or in a greenhouse (9 × 13 m) at elevated CO2 with fogging (ECF), respectively, at the National Agriculture and Food Research Organization, Institute of Vegetable and Tea Science, Taketoyo, Aichi, Japan. In the AMC greenhouse, the temperatures for natural ventilation and heating initiation were set at 25 and 13°C, respectively, although the humidity and CO2 level were not controlled. In the ECF greenhouse, the temperatures for natural ventilation and heating initiation were set at 25 and 15°C, respectively. The daytime CO2 levels in ECF at 0–29, 30–95, and 96–165 days after transplanting (DAT) were maintained at 1500, 1000, and 600 μmol·mol−1, respectively. A fogging system (FG-2; Matsusaka Engineering, Tokyo, Japan; power 2.2 kW) was used during the daytime in ECF for humidification, maintaining 80% relative humidity (RH). Air temperature, RH, and solar radiation inside and outside the greenhouses were measured at 1 min intervals by the Ubiquitous environmental control system (Yasuba et al., 2013). Vapor pressure deficit (VPD) was obtained using Tetens’s equation and RH. Planting densities in AMC and ECF were 2.46 and 2.78 plants·m−2, respectively. The spacings were 60 cm between rows within the double rows in both AMC and ECF, and 220 or 200 cm between double rows in AMC or ECF, respectively. The plants in both AMC and ECF were supplied with a commercial nutrient solution (Otsuka-A; Otsuka AgriTechno, Tokyo, Japan), which consisted of 9.3 mM NO3−, 4.3 mM K+, 4.1 mM Ca2+, 1.5 mM Mg2+, 0.9 mM H2PO4−, 2.7 mg·L−1 Fe, 1.2 mg·L−1 Mn, 0.51 mg·L−1 B, 0.09 mg·L−1 Zn, 0.03 mg·L−1 Cu, and 0.03 mg·L−1 Mo, adjusted to an electrical conductivity of 2.0 to 2.4 dS·m−1 as the plants grew. The interval of nutrient solution supply was controlled based on outdoor solar radiation. The daily draining percentage was maintained at 20% to 30% of the total quantity of nutrient solution supplied. The drainage was not reused for cultivation. According to standard Dutch practice, the plants were trained vertically at ca. 3 m height and lowered as they grew, and their old leaves that were colored yellow were pruned. The pruned auxiliary buds and leaves were excluded from the measurement. Flowers were pollinated by bumble bees. The number of fruit per truss was not adjusted by pruning. The experiment was conducted in 3 plots. The cultivars were set randomized in each plot in each greenhouse. In each plot, 20–24 or 18 plants per cultivar were planted into double rows in AMC or ECF, respectively. These plants were surrounded by 65 or 60 border plants per cultivar per row with both sides in AMC or ECF, respectively. The border plants were also excluded from the measurement.
We harvested all mature fruit from 8 plants per cultivar combination in each plot 3 times per week and measured their fresh and dry weights. We also measured leaf (leaf blade + petiole) area using an LI-3100C leaf-area meter (LI-COR, Lincoln, NE, USA) and the fresh and dry weights of leaves, stems, and all fruit of 2 or 4 plants destructively sampled per cultivar per plot per greenhouse at 3, 67, 94, and 171 DAT in AMC, and at 3, 60, 87, and 165 DAT in ECF. Fraction of dry matter distribution to fruit (FDF) was obtained as a quotient of the total dry weight of fruit divided by TDM. Mature and immature fruit were measured separately. To avoid the effects of this sampling on the remaining plants and on their light interception, we used 2 plants and 2 adjacent guard plants per cultivar per plot, in each measurement in the 2nd and 3rd destructive samplings. The individual-leaf photosynthetic rate and stomatal conductance in ECF and AMC were measured at 69 and 74 DAT, respectively, with a portable photosynthesis system (LI-6400; LI-COR) at PPFD of 1500 μmol·m−2·s−1 and 1000 μmol·mol−1 CO2. The measurements were conducted between 11:00 and 14:30 using 2 mature leaves (without shading by any other leaves) among the 3rd to 5th leaves from the top per plant, with the use of 2 plants in each cultivar per plot. Leaf temperatures (mean ± SD) during the measurements in ECF and AMC were 31.2 ± 1.2 and 28.0 ± 1.0°C, respectively.
To obtain the light-extinction coefficient within the plant canopy for each cultivar, we measured PPFD using a 1-m-long PPFD sensor (LI-191SA; LI-COR) at 6 different heights within the closed plant canopies in ECF and AMC at 83 and 96 DAT, respectively. PPFD above the plant canopy was also measured with a PPFD sensor (LI-190SA; LI-COR) and recorded using a datalogger (GL220; Graphtec, Yokohama, Japan). The individual-leaf area of each cultivar was obtained using the regression equation by the same method as Higashide et al. (2012). The linear regression equations were obtained by destructive sampling of 6 plants per cultivar in ECF and AMC at 60 and 94 DAT, respectively. The cumulative leaf area index (LAI) at each of the 6 heights within the canopy was calculated from the individual-leaf area and the number of leaves at that height. The light-extinction coefficient was obtained using the equation by Monsi and Saeki (1953, 2005) as the slope of a logarithmic regression of PPFD against cumulative LAI at the 6 heights. LUE was calculated as the slope of a linear regression of the cumulative TDM as a function of the integrated interception of photosynthetically active radiation (PAR) on the 4 sample dates by the same method as used by Higashide and Heuvelink (2009). Greenhouse transmissivity and the fraction of PAR were assumed to be 60% and 50% of global radiation, respectively (Kurata, 1994; Ohtani, 1997). Daily PAR intercepted by the plants of each cultivar in each greenhouse was calculated from LAI and the corresponding light-extinction coefficient. The number of trusses having immature fruits per plant was obtained as a quotient of the number of immature fruits per plant divided by the average number of fruit per truss. The influences of 2 environmental conditions: AMC and ECF, 3 cultivars: Ab, Af, and My, and their interaction on the growth characteristics were analyzed by two-way ANOVA. Pearson’s correlations among the various yield components were also investigated based on the report by Higashide and Heuvelink (2009).
Daily cumulative PAR in AMC and ECF fluctuated similarly but increased during the experimental period (Fig. 1A). Daily average temperature was higher in ECF than in AMC from November to March. The temperatures in AMC and ECF increased with almost the same tendency from April to the end of the experiment. Daytime (8:00–16:00) CO2 level in AMC was ca. 500 μmol·mol−1 during the experiment. The daytime CO2 level in ECF fluctuated at ca. 1500 μmol·mol−1 at 0–30 DAT, and decreased to ca. 1000 μmol·mol−1 at 30–96 DAT and to ca. 600 μmol·mol−1 from 96 DAT to the end of the experiment. Average temperature and CO2 level during the experimental period (mean ± SD) were 16.7 ± 2.9°C and 509 ± 26 μmol·mol−1 in AMC, and 18.9 ± 1.4°C and 745 ± 179 μmol·mol−1 in ECF, respectively. On a clear day, although the night temperature in ECF was higher than that in AMC, temperatures in the daytime in AMC and ECF were almost the same, ca. 25°C (Fig. 1B). VPD in the daytime in AMC was extremely high (> 2 kPa; < 30% RH) although nighttime VPD in AMC was low (0.2–0.3 kPa; ca. 80% RH). Daytime VPD in ECF fluctuated in the range of 0.2–1.5 kPa (ca. 50–90% RH), and nighttime VPD in ECF was 0.6–0.8 kPa (ca. 60% RH).

Daily cumulative photosynthetically active radiation (PAR), average air temperature (Tmp) and daytime (8:00–16:00) CO2 concentration during the experimental period (A), and diurnal change in air temperature and vapor pressure deficit (VPD) (B; measured on a clear day; March 5, 2011) in the AMC greenhouse (ambient CO2 without fogging) or the ECF greenhouse (elevated CO2 with fogging). DAT: days after transplanting.
Fresh-weight (FW) fruit yield in ECF was significantly higher than that in AMC: 1.15–1.45 times as large as that in AMC (Fig. 2). In terms of FW yield, that for My was significantly lower than for Af in AMC, and was lower than for Ab and Af in ECF. In each cultivar, FW yield in ECF was also significantly higher than that in AMC by Bonferroni posttest. Fruit dry-matter content (DMC) did not differ significantly between ECF and AMC. DMC in Af was significantly lower than those in the other cultivars in both ECF and AMC. There was also no significant interaction of environment × cultivar for both the yield and the DMC. The number of leaves, stem length, LAI, and specific leaf area (SLA) were significantly higher in ECF than those in AMC (Table 1). They also differed significantly among the cultivars. In both ECF and AMC, My was significantly higher in terms of the number of leaves and lower in terms of SLA than the other cultivars, except for Af in AMC. Af was significantly lower in terms of LAI than the others. There was no significant interaction of environment × cultivar for them. In each cultivar, the number of leaves, stem length, and LAI in ECF were also significantly higher than those in AMC by Bonferroni posttest. In Af, SLA in ECF was also significantly higher than that in AMC by the posttest. Leaf and stem dry weight (DW), DW fruit yield, and TDM in ECF were significantly higher than those in AMC. Although they differed significantly among the cultivars, there was no significant interaction of environment × cultivar for them. DW yield in ECF increased 1.15–1.45 times compared with that in AMC. Ab was significantly higher in terms of DW yield than My in ECF. TDM in ECF also increased 1.39–1.46 times compared with that in AMC.
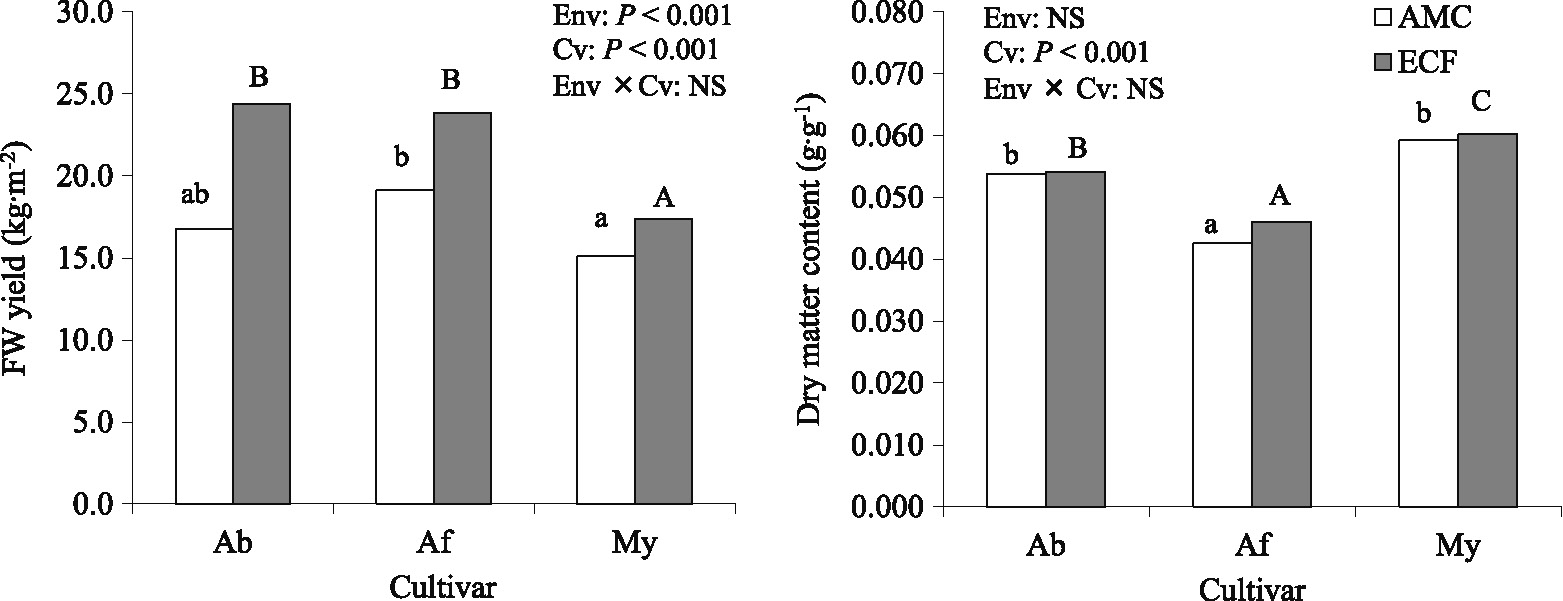
Fresh-weight (FW) fruit yield per area, and fruit dry matter content of the 3 Japanese cultivars (Ab: Asabiyori 10; Af: Aichi Fast; My: Momotaro York) grown at ambient CO2 without fogging (AMC) or elevated CO2 with fogging (ECF) at 171 or 165 days after transplanting, respectively. The results of two-way ANOVA (n = 12) with environmental condition (Env) and cultivar (Cv) as independent variables and their interaction (Env × Cv) for each dependent variable are shown in each panel. NS: not significant (P ≥ 0.05). Different letters indicate significant differences for the same environmental condition (P < 0.05; by Bonferroni posttest).

Number of leaves, stem length, leaf area index (LAI), specific leaf area (SLA), leaf and stem dry weight (DW), DW fruit yield, and total above-ground dry matter (TDM) of the 3 Japanese cultivars grown at ambient CO2 without fogging (AMC) or elevated CO2 with fogging (ECF) at 171 or 165 days after transplanting, respectively.
TDM per cumulative intercepted PAR in ECF was higher than that in AMC in each cultivar (Fig. 3). Thus, LUE represented as the slopes in this figure were significantly higher in ECF than those in AMC: 1.5–1.6 times as large as those in AMC. Although My was significantly higher in LUE than the others in AMC based on 95% confidence intervals, there was no significant difference in LUE among the cultivars in ECF. The maximum photosynthetic rate of individual leaves did not differ significantly between AMC and ECF (Table 2). The rate in My was significantly higher than that in the other cultivars in both AMC and ECF. Stomatal conductance was significantly higher in AMC than that in ECF. As there were also significant differences in the conductance among the cultivars, the conductance in Af was significantly lower than that in the others in AMC. There was a significant interaction of environment × cultivar for both the rate and the conductance. Although the light-extinction coefficient differed significantly among the cultivars, there was no significant difference in the coefficient between AMC and ECF, except for that in Ab. The coefficient in Ab was significantly higher in ECF than that in AMC.

Total above-ground dry matter as a function of cumulative intercepted PAR in the 3 Japanese tomato cultivars (Ab: Asabiyori 10; Af: Aichi Fast; My: Momotaro York) grown at ambient CO2 without fogging (AMC-) or elevated CO2 with fogging (ECF-). The slopes of the regression lines, namely, light use efficiency (95% confidence intervals), of AMC-Ab, AMC-Af, AMC-My, ECF-Ab, ECF-Af, and ECF-My are 2.67 (2.53–2.81), 2.68 (2.52–2.84), 2.85 (2.75–2.95), 4.36 (4.35–4.38), 3.97 (3.55–4.39), and 4.16 (4.04–4.28), respectively (R2 = 0.999–1.0, P < 0.01).

Maximum photosynthetic rate, stomatal conductance, and light-extinction coefficient of the 3 Japanese cultivars grown at ambient CO2 without fogging (AMC) or elevated CO2 with fogging (ECF).
FW fruit yield was significantly and positively correlated with DW fruit yield (r = 0.89, P < 0.05), although there was no significant correlation between FW yield and DMC (Table 3). DW yield was significantly and positively correlated with TDM (r = 0.89, P < 0.05), although there was no significant correlation between DW yield and FDF. DW yield was also significantly and positively correlated with LUE and the number of fruit per plant (r = 0.89 and 0.95, P < 0.05 and 0.01, respectively). TDM was significantly, positively, and highly correlated with LUE (r = 0.98, P < 0.01), although there was no significant correlation between TDM and the cumulative intercepted PAR. TDM was also significantly and positively correlated with LAI and the number of fruit per plant (r = 0.91 and 0.89, P < 0.05, respectively). Although LUE was also significantly and positively correlated with LAI and the number of fruit per plant (r = 0.83 and 0.95, P < 0.05 and 0.01, respectively), there was a significant correlation neither between LUE and the light-extinction coefficient, nor between LUE and the maximum photosynthetic rate.

Correlations (Pearson’s r coefficient) between the yield components and characteristics of dry matter production of the 3 Japanese tomato cultivars grown at ambient CO2 without fogging and elevated CO2 with fogging.
Inflorescence appearance per plant was significantly higher in ECF than that in AMC (Table 4). My was significantly higher in terms of the inflorescence appearance than the other cultivars in both AMC and ECF, and reached the 21st inflorescence appearance in ECF. There was a significant interaction for environment × cultivar for the inflorescence appearance. The numbers of flowers per inflorescence or per plant differed significantly between ECF and AMC. Such numbers also differed significantly among the cultivars: the number in Af was significantly higher than those in the other cultivars. Although there was no significant difference in the fruit set ratio between AMC and ECF, the ratio differed significantly among the cultivars. Af was significantly lower in terms of the ratio than the others in both AMC and ECF. The number of harvested fruit per truss also differed significantly between AMC and ECF, or cultivars, but there was no significant interaction of environment × cultivar. My was significantly lower in terms of the number of fruit per truss than the other cultivars in both AMC and ECF, except for Ab in AMC. Although the number of fruit per plant was significantly higher in ECF than that in AMC, there was no significant difference in the fruit number among the cultivars. FW per fruit decreased significantly in ECF. In both AMC and ECF, My was significantly lower in terms of the fruit FW than the other cultivars. There was no significant interaction of environment × cultivar for the number of flowers, fruit set ratio, number of harvested fruit, and FW per fruit. In each cultivar, inflorescence appearance, the number of flowers per plant, the number of fruit per plant, and the fruit FW also differed significantly between ECF and AMC by Bonferroni posttest, except for the number of flowers per plant in My. In each cultivar, the number of flowers per inflorescence and the number of fruit per truss did not differ significantly between AMC and ECF by Bonferroni posttest, although there were significant differences between AMC and ECF by two-way ANOVA.

Number of flowers per inflorescence and plant, fruit set ratio: fruits/flowers, number of harvested fruit, and fresh weight (FW) per fruit of the 3 Japanese cultivars grown at ambient CO2 without fogging (AMC) or elevated CO2 with fogging (ECF) at 171 or 165 days after transplanting, respectively.
FDF differed significantly among the types of environment, cultivars, or the interaction of environment × cultivar (Fig. 4). Although there was no significant difference in FDF in both Ab and Af between AMC and ECF, FDF in My was significantly lower in ECF than that in AMC. Although there was no significant difference in the number of trusses having immature fruit per plant between AMC and ECF, the number differed significantly among the cultivars (Fig. 4). The number of trusses having immature fruit in My in ECF increased significantly compared with those in the others. The number of trusses having immature fruit in My in ECF reached ca. 7 trusses per plant, and was ca. 1.3 trusses per plant (ca. 5 fruit per plant) larger than that in AMC.

Fraction of dry matter (DM) allocation to fruit, and number of trusses having immature fruit per plant of the 3 Japanese cultivars (Ab: Asabiyori 10; Af: Aichi Fast; My: Momotaro York) grown at ambient CO2 without fogging (AMC) or elevated CO2 with fogging (ECF) at 171 or 165 days after transplanting, respectively. The results of two-way ANOVA (n = 12) with environmental condition (Env) and cultivar (Cv) as independent variables and their interaction (Env × Cv) for each dependent variable are shown in the panel. NS: not significant (P ≥ 0.05). Different letters indicate significant differences (P < 0.05; by Tukey’s multiple-comparison test).
Tomato yield consists of yield components hierarchically (Higashide and Heuvelink, 2009). FW yield, consisting DW yield and DMC, was improved by the elevated CO2 and fogging (Fig. 2). Since DMC was not affected by the elevated CO2 and fogging, FW yield improvement mainly consisted of the increase in DW yield (Table 1). Similarly, DW yield improved mainly due to the increase in TDM since FDF was not increased by the elevated CO2 and fogging (Table 1; Fig. 4). The increase in TDM resulted from higher LUE, since intercepted PAR was changed little by the elevated CO2 and fogging (Fig. 3). These results were also supported by the correlations between the yield components and characteristics of dry matter production (Table 3), and coincided with a previous report on Dutch cultivars in which yield improvement was described as being caused by the increase in LUE (Higashide and Heuvelink, 2009). This increase in LUE by elevated CO2 has already been reported in tomatoes (de Koning, 1997). Nederhoff (1994) reported that CO2 elevation increased LUE by 15% per 100 μmol·mol−1 CO2. On the basis of her report, we estimated that LUE was increased from 2.67–2.83 in AMC to 3.97–4.36 in ECF (Fig. 3) by ca. 300 μmol·mol−1 CO2 elevation. Indeed, the average CO2 concentration including that at nighttime measured in ECF was 745 μmol·mol−1 during the experimental period, although the concentration in the daytime was set from 1500 to 600 μmol·mol−1 CO2.
Although LUE is influenced by photosynthetic rate and light-extinction coefficient, no significant difference in the maximum photosynthetic rate was found between AMC and ECF in each cultivar (Table 2). Matsuda et al. (2013) reported on the difference in photosynthetic characteristics between Dutch and Japanese cultivars. They observed no difference in the maximum photosynthetic rate between Ab and My, although My was higher in terms of this rate than Ab in both AMC and ECF in this experiment. Besford (1993) reported that the photosynthetic rate of plants grown at 1000 μmol·mol−1 was reduced significantly compared with that at 340 μmol·mol−1. This photosynthetic reduction is known to involve acclimation to elevated CO2 for a long time (Besford et al., 1990; van Oosten et al., 1995; Yelle et al., 1990). However, no reduction of the maximum photosynthetic rate by elevated CO2 and fogging was observed in this experiment (Table 2). In addition, Li et al. (2013) reported that elevated CO2 increased the dark respiration of tomato leaves during the nighttime. However, TDM and LUE clearly increased in this experiment, although several reports indicated negative effects of long-term CO2 elevation.
Transpiration of tomato plants increased when VPD increased, that is, humidity decreased (Jolliet and Bailey, 1992). However, the transpiration and yield of tomatoes were decreased by high humidity at 0.2–0.3 VPD (Bakker, 1990; Jolliet et al., 1993). Although VPD ranged from ca. 0.2 to ca. 1.0 kPa in their experiments, VPD reached higher than 2.0 kPa in AMC in our experiments (Fig. 1B). Extreme low humidity closed the stomata of tomato leaves, and thereby decreased transpiration and photosynthetic rates (Stangellini and Bunce, 1993). Humidification by fogging mitigated the closing stomata and thereby improved the tomato yield in low-humidity areas such as Mediterranean coastal areas (Romero-Aranda et al., 2002). Humidification in these areas also increased the viability of tomato pollen grain (Harel et al., 2014). Similarly to the finding in these areas, in our experiment, humidity in the AMC greenhouse decreased markedly during the daytime. On the other hand, the fogging in the ECF greenhouse avoided this extremely low humidity (Fig. 1B) and thus contributed to the improvement of yield.
In many studies, SLA in tomatoes and in other plants was decreased by elevated CO2 (Nederhoff, 1994; Poorter, 1993; Takahashi et al., 2012). However, SLA was increased by the elevated CO2 and fogging in our study (Table 1), which indicated leaf thinning. In our experiment, the humidity during the daytime and plant density in ECF were higher than those in AMC. These conditions might compensate for the decrease in SLA due to CO2 elevation. Since SLA reflects leaf thickness, it might be assumed that SLA influences light penetration in the tomato canopy. Moreover, the increases in leaf area and weight, and LAI by the elevated CO2 and fogging (Table 1) might also have influenced the light penetration morphologically. However, no significant difference in the light-extinction coefficient between AMC and ECF was observed, except for Ab (Table 2). This result suggests little influence of the elevated CO2 and fogging on the light penetration in the canopy. Consequently, the light-extinction coefficient may also have little influence on the increase in LUE.
The yield and TDM were clearly increased by the elevated CO2 and fogging, although there was no significant effect on the maximum photosynthetic rate or light-extinction coefficient. Accordingly, elevated CO2 and fogging may have little influence on the potential of leaf photosynthesis and on the light penetration in the tomato canopy. Although vegetative growth such as leaf growth was increased by the elevated CO2 and fogging, no negative effect on the light penetration in the tomato canopy was observed in our experiment.
Difference in effects of elevated CO2 and fogging on the yield among cultivarsThe increases in TDM and in LUE by ECF did not differ significantly among the cultivars. However, Ab was significantly higher in terms of the fruit yield than My in ECF, although there was no significant difference between Ab and My in AMC (Fig. 2). This result indicated that the effects of ECF differed depending on the cultivars. Differences in tomato yield by elevated CO2 and fogging between cultivars were also reported by Yasuba et al. (2011). Rogers and Dahlman (1993) reported an increase in dry matter allocation to reproductive organs in corn and maize plants by CO2 elevation as an increase in TDM. Nederhoff (1994) also reported that CO2 elevation increased FDF in cucumber and sweet pepper plants, and maintained it in tomato plants. An increase in FDF by CO2 elevation was reported by Tripp et al. (1991) in 7 tomato cultivars. Similarly to these previous reports, no reduction of FDF by ECF was observed in Ab and Af (Fig. 4). In Ab in ECF, FDF, and TDM increased 0.99 and 1.46 times compared with those in AMC, respectively. Consequently, DW yield increased 1.46 times compared with that in AMC, which suggests that the increase in TDM by ECF contributed directly to the yield increase. However, FDF by ECF decreased in My (Fig. 4). Although TDM in My increased by ECF 1.39 times compared with that in AMC, DW yield increased only 1.20 times. The yield increment in My was mitigated compared with those in the others, Ab and Af. This mitigation of yield increment was caused by a reduction of FDF in ECF: 0.85 times as large as that in AMC. This characteristic shown in My might suggest that CO2 elevation promoted vegetative growth rather than the fruit yield in Japanese cultivars.
Reduction of dry matter allocation to fruit in ‘Momotaro York’FDF is determined by the sink strength of fruit, which is contributed to strongly by the number of fruit per truss in tomatoes (Hevelink, 1996; Hevelink and Marcelis, 1989). In this experiment, no significant difference in the number of fruit per truss or the fruit set ratio was observed between AMC and ECF in My (Table 4). Accordingly, the reduction of FDF in My may be independent of the number of fruit per truss. Matsuda et al. (2011) reported that the potential fruit growth rate of My in elevated CO2 was lower than that in ambient CO2, although the growth rates of Dutch cultivar were comparable between elevated and ambient CO2. Although the reason for this difference was unclear, the decrease in potential fruit growth rate due to elevated CO2 in My indicated the reduction of sink strength per fruit. Although we did not measure the potential growth rate, ECF might reduce the sink strength per fruit in My, and thereby decrease FDF.
The number of leaves was increased by CO2 elevation (Hicklenton and Jolliffe, 1978). Leaf unfolding rate was also affected by temperature (de Koning, 1992, 1994). A decrease in temperature by using fogging caused a delay of truss appearance (Kinomoto et al., 2013). In this experiment, the air temperatures in ECF were higher than those in AMC (Fig. 1). Thus, the numbers of leaves and inflorescences were increased significantly by ECF in all cultivars (Tables 1 and 4). However, no significant increase in the number of trusses having immature fruit was observed by ECF in both Ab and Af (Fig. 4). In contrast, the number of trusses having immature fruit was significantly higher in ECF in My. My in ECF had ca. 1.3 trusses having immature fruit, which was more than that in AMC. Consequently, the number of immature fruit per plant was increased by the higher leaf and inflorescence appearance in My. We assumed that the assimilation products due to this increase in leaves were insufficient to allocate to the increased number of fruit, and thus reduced FDF in My. This reduction of FDF by the increase in the number of immature fruit per plant might also be linked to the suggested effectiveness of CO2 elevation on Japanese cultivars. Although the maturation rate of fruit was not measured in this experiment, Takada (1976) reported that CO2 elevation reduced the respiration of tomato fruit and thereby the maturation rate. Although the shelf-life of tomatoes was shortened by high humidity at postharvest (Bakker, 1990), the influence of humidity on growing fruit was unclear. ‘Momotaro’-type cultivars have different characteristics, such as a higher level of soluble solids per dry matter in fruit, compared with the previous cultivars in Japan (Higashide et al., 2012). Our results may indicate another trait of ‘Momotaro York’, in that FDF was reduced by elevated CO2 and fogging (Fig. 4).
Decrease in fruit size by elevated CO2 and foggingThe fruit weight per fruit, namely, fruit size, was decreased significantly by ECF, although both FW yield and the number of fruit increased (Table 4). We assumed that this decrease in fruit size was caused by several factors as follows. One is that the number of fruit in ECF increased more, 1.33–1.69 times that in AMC, compared with the increase in TDM, 1.39–1.46 times that in AMC, although FDF was maintained or decreased by ECF. The increase in the number of fruit by ECF was mainly determined by the higher number of flowers per plant, since there was no significant difference in the fruit set ratio (Table 4). The higher number of flowers per plant was determined by both the higher inflorescence appearance and the higher number of flowers per inflorescence. Humidity and temperature would also influence the decrease in fruit size by ECF. Bakker (1990) reported on a decrease in fruit size by high humidity. The maturation rate of tomato fruit depends on the temperature (Adams, 2002; Adams and Valdes, 2002). Since ECF was 2.2°C higher in terms of average air temperature than AMC during the whole experimental period, ECF promoted fruit maturation, and thereby decreased the duration from anthesis to harvest and the fruit size. Temperature affects both leaf appearance and fruit maturation rate, and is relatively easy to control in a greenhouse. To avoid the decrease in fruit weight per fruit at elevated CO2 and fogging, we suggest controlling the temperature at a lower level in greenhouses.
ConclusionWe concluded that the fruit yield and TDM were improved by the elevated CO2 and fogging in the 3 Japanese cultivars, and that these increases were mainly determined by the increase in LUE. The elevated CO2 and fogging increased the number of harvested fruit but decreased fruit weight per fruit, namely, fruit size, in the 3 cultivars. Although leaf area, LAI, and fresh and dry weights of leaves and stem were increased by the elevated CO2 and fogging, no negative effect such as a change in light-extinction coefficient was observed. The maximum photosynthetic rate of individual leaves was also not changed by the elevated CO2 and fogging. Similarly to many reports, no decrease in FDF was observed by the elevated CO2 and fogging in ‘Asabiyori 10’ and ‘Aichi Fast’. The increase in TDM by higher LUE therefore contributed directly to the fruit yield increase in these 2 cultivars. However, FDF in ‘Momotaro York’ was decreased significantly by the elevated CO2 and fogging. Thereby, the yield increase by the elevated CO2 and fogging diminished in ‘Momotaro York’ in spite of the increase in TDM. Although the leaf and inflorescence appearances were increased by the elevated CO2 and fogging in the 3 cultivars, no increase in the number of trusses having immature fruit per plant was observed by the elevated CO2 and fogging in ‘Asabiyori 10’ and ‘Aichi Fast’. However, in ‘Momotaro York’, the number of trusses having immature fruit was increased significantly by the elevated CO2 and fogging. These characteristics shown in ‘Momotaro York’ may diminish the effects of elevated CO2 and fogging on fruit yield. On the basis of these characteristics shown in ‘Momotaro York’, we inferred that some growers doubted the effectiveness of elevated CO2 and fogging on Japanese cultivars.