2015 Volume 84 Issue 3 Pages 269-276
2015 Volume 84 Issue 3 Pages 269-276
This study was conducted to demonstrate the seasonal change of floral organ number and morphology, and the effect of growth temperature on floral morphology in double-flowered cyclamen with petaloid-stamens. In plants grown under seasonal variable temperature, floral organ number and morphology in petaloid-stamen type of double flower changed as time passed, but the degrees of such changes differed depending on the line; there were two types, namely, “variable” and “relatively stable”, in terms of the number of outer and inner petaloid-stamens throughout the flowering period. In the plants grown under different constant temperatures, the rate of complete petals (petaloid-stamens) to the total organs in whorl 3 was greatest at 15°C, followed by the values at 25°C and 20°C. In contrast to complete petals, the rate of incomplete petals (petaloid-stamens) to the total organs in whorl 3 was greatest at 20°C, followed by the values at 25°C and 15°C. The rate of stamen-like organs to the total organs in whorl 3 did not differ significantly among treatments, and the rate of stamens to the total organs in whorl 3 was suppressed only at 25°C. Although the total numbers of stamens and stamen-like organs were similar at 15°C and 20°C, the developed positions of stamen-like organs and stamens were significantly different between 15°C and 20°C. Additional organs including stamens were produced inside the petaloid-stamen at 15°C, while restoration of the stamen was induced at 20°C. In conclusion, floral morphology shows seasonal change, and growth temperature affects the petaloidy of stamen in double-flowered cyclamen. However, the effect on petaloidy differs according to the line.
The genus Cyclamen is a bulbous plant in the Primulaceae, with 22 species, being mainly native to the Mediterranean region (Grey-Wilson, 2002). As C. persicum has various flower colors, patterns, and shapes, it is one of the most popular flowering plants in Europe and Japan. In particular, it is a major winter pot flower in Japan. It is said that the production of this flower in Japan started in the early 1900s. Its production then increased year by year, and the largest total annual production was approximately 22.9 million pots in 2002 (Ministry of Agriculture, Forestry and Fisheries of Japan, 2014; http://www.e-stat.go.jp/SG1/estat/List.do?bid=000001024933&cycode=0). Although its level of production is the greatest among pot flowers, its price and production scale have decreased in recent years. Thus, new cultivars with notable traits enhancing the ornamental value are required.
“Double flower” is a very important characteristic for ornamental plants. In cyclamen, double flowers with the transformation from stamens to petals were produced by natural mutation. This type of double flower has advantages that enhance the ornamental value of the flower: large flower size and prevention of pollen dust on petal and leaf surfaces. Despite these advantages of petaloidy, there is a serious problem in double flowers with petaloid-stamens. Although cyclamen is usually propagated by seeds, it is impossible to use pollen of double-flowered cyclamens with petaloid-stamens. Additionally, it has been reported that crosses between double and single flowers result in the segregation of single and double flowers in the progeny (Terakawa, 2012). This problem is a key obstacle to commercial production and genetic research. As mentioned above, except for a few cultivars, clonal propagation is used for commercial production in double-flowered cyclamen. However, clonal propagation using tissue culture has major disadvantages in terms of the cost and proliferation efficiency in cyclamen.
In many flowering plants, floral morphogenesis can be generally explained by the “ABC-model” (Coen and Meyerowitz, 1991). Even in cyclamen, double-flower formation follows this model, and C-class (AGAMOUS-like) gene suppression causes petaloidy of the stamen (Mizunoe et al., 2015). On the other hand, double-flowered cyclamen with petaloid-stamen has not only complete and incomplete petals but also stamens within a plant. However, the key factors influencing the variation of petaloid-stamen morphology in double-flowered cyclamen are unknown.
The effect of temperature on floral morphology has been investigated in many species of flowering plants. It has been reported that the petaloidy level of the stamen in the flower of Daucus carota is accelerated as temperature increases (Eisa and Wallance, 1969). In addition, stamens in blackberry flowers are replaced by petal-like structures at high temperature (Stanton et al., 2007). These reports mention that high temperature induces petaloidy of stamens, whereas other reports mention that high temperature induces stamen formation. In the model plant, Arabidopsis thaliana, apetala-2 mutant shows various phenotypes depending on the temperature: staminoid petals and stamens are formed at high temperature in the place where petals should form (Bowman et al., 1989). It has also been reported that stamen development is thermosensitive, that is, high temperature results in anther development in some antherless male sterile cultivars of lily (Sato and Miyoshi, 2006, 2007).
In addition, it has also been reported that temperature affects the number of floral organs. Plants grown at low temperature produce a greater number of organs than those at intermediate and high temperatures in Lycopersicon esculentum (Sawhney, 1983). Lateral stamen number of Cardamine hirsuta (Brassicaceae) flower is unstable even within an inflorescence, being affected by growth temperature (Matsuhashi et al., 2012). Furthermore, low and high temperatures cause an increase of petal number via petal formation in the center of the flower and the receptacle, respectively, in carnation (Dianthus caryophyllus) (Garrod and Harris, 1974). These previous studies indicate that the influence of temperature on floral development shows variation depending on the plant species.
In petaloid-stamen-type double-flowered cyclamen, clarification of the effect of growth temperature on floral morphology and the establishment of artificial control of petaloidy level will lead to stable production. Additionally, if the artificial control of petaloidy of stamens is possible in double-flowered cyclamen, investigation of the inheritance of the double-flower trait and the breeding of seed-propagated double-flowered cultivars will be accelerated using pollen grains from double-flowered cultivars. This study was thus conducted to demonstrate the seasonal change of floral organ number and morphology, and the effect of growth temperature on floral morphology in double-flowered cyclamen with petaloid-stamens.
For investigation of the seasonal change of floral morphology of double flowers, two groups of F1 progeny generated by crosses between double flowers with complete petaloid-stamen and incomplete petaloid-stamen were supplied. The plants of first group were two years old and consisted of three lines: eight plants in H23-331 (Fig. 1), ten plants in H23-332 and twelve plants in H23-333. The plants of second group were one year old and composed of two lines: five plants each in H24-122 and H24-131 lines. The plants of the first group were planted in a plastic pot (D = 16 cm, H = 13 cm) filled with approximately 1100 g of soil consisting of a 2:1:1 mixture (v/v/v) of leaf mold, small-sized akadama soil (red clay granular), and small-sized bora soil (air-permeable soil delivered from pumice). Seven out of twelve plants in H23-333 were planted in a plastic pot (D = 13 cm, H = 8.5 cm) filled with approximately 370 g of mixed culture soil consisting of mainly peat moss, perlite, small-sized akadama soil (red clay granular), and vermiculite, which was the same condition as for the plants in the two H24 lines in the second group.
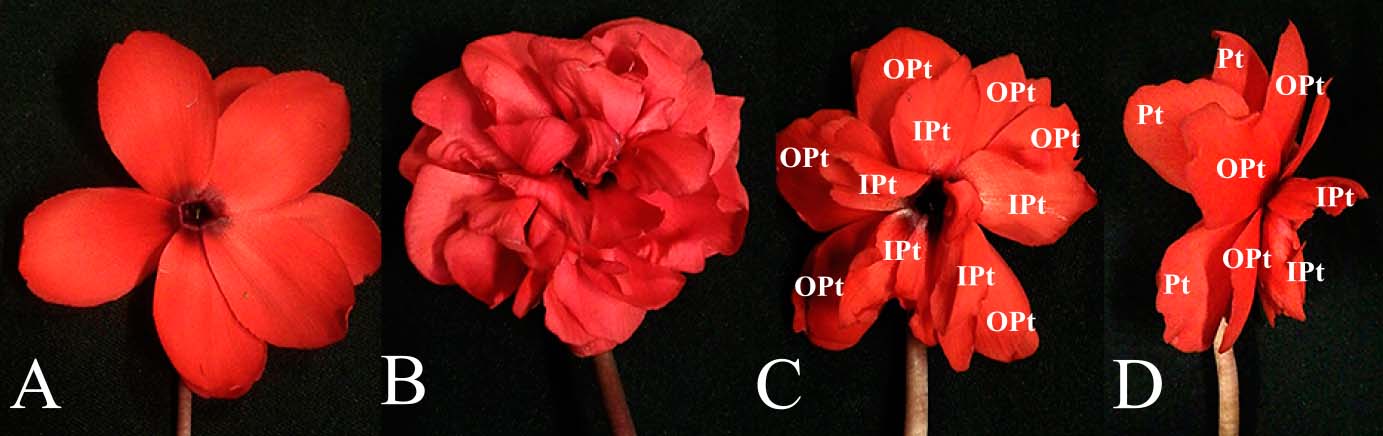
Morphological variation of double flowers in H23-331 line. (A) A typical double flower. The flower has five normal petals and five petaloid-stamens. (B) Double flower with numerous petals. The flower has six or more petals inside the whorl of normal petals. (C) (D) Categorization of petals. Pt: normal petals. OPt: outer petals developed close to normal petal. IPt: inner petal developed between outer petals and a pistil.
To clarify the effect of temperature on floral morphology of double flower, petaloid-stamen-type plants of the clonal double-flowered cultivar ‘Wink Pink II’ planted in a plastic pot (D = 14 cm, H = 14 cm) containing about 650 g of culture soil were obtained from a grower. Three plants were supplied for each temperature treatment in this study.
Time-dependent change of floral morphology of double flowerIn the first group, plants were grown in a greenhouse by Kage-shinkouen Co., Ltd. (Kurume, Fukuoka, Japan). All plants except for seven plants in H23-333, and these seven plants were transferred into a greenhouse (maintained at over 10°C, natural photoperiod) in Kyushu University (Fukuoka, Japan) on Sep. 28 and on Dec. 20, 2012, respectively. The number of organs and floral morphology were investigated from Feb. 25 to Mar. 25, 2013 (for five weeks). In the second group, although the cultivation history was almost the same as that in the first group, the transportation date and investigation period were Apr. 25, 2013 and from Apr. 26 to June 28, 2013 (for ten weeks), respectively. Floral organs were classified into sepal, normal petal closed to sepal (Pt), outer petal (OPt) occurring next to a normal petal, inner petal (IPt) produced inside the whorl of outer petals, stamen (S), and pistil. The number and morphology of normal petals, outer and inner petals, and stamens were investigated. During the investigation period, the plants were irrigated equally as necessary, and no additional fertilizer was applied.
Effect of temperature on floral morphology of double flowerPlants were maintained at three different temperatures, 15°C, 20°C, and 25°C, under R.H. 70%, with a natural photoperiod, in a phytotron at Kyushu University from Nov. 1, 2012. Flowers with sufficiently elongated stalks and at/after anthesis were removed on Nov. 2, 2012. Completely opened flowers were sampled every week from Dec. 19, 2012, to Mar. 6, 2013 (for twelve weeks), and the floral morphology of petaloid-stamen in whorl 3, length of flower stalk, and length and width of normal petals in whorl 2 were investigated (length and width of the petaloid-stamen could not be measured consistently because of its variable morphology). When the floral morphology of the petaloid-stamen in whorl 3 was investigated, the organs that developed between normal petals and a pistil were classified into four categories depending on their morphology (Fig. 2). The first is complete petal (petaloid-stamen) (Fig. 2A, B), the second is incomplete petal (petaloid-stamen) with deformation and miniaturization, among others (Fig. 2C, D), the third is a stamen-like organ with a partial petaloid structure and/or without pollen grains (Fig. 2E, F), and the fourth is a complete stamen with germinable pollen grains (Fig. 2G–I). During the investigation period, the plants were irrigated equally as necessary, and no additional fertilizer was given.

Morphological variation of double flowers in ‘Wink Pink II’. A flower with complete petal (A) (B), incomplete petal (C) (D), stamen-like organ (E) (F), stamen (G) (H) (I), and stamen formed inside petaloid-stamens (J) (K) (L). (B) (D) (F) (H) (K) Magnifications of the pictures to the left. (I) and (L) Longitudinal sections of (G) and (J), respectively. Pt: normal petal, PS: petaloid-stamen, S: stamen, P: pistil.
Focusing on organ number, all lines were categorized into two groups. The first is “the lines with flowers in which many outer and inner petals were produced during the early flowering period, but the number of petals gradually decreased as the flowering period progressed” and the second is “the lines with flowers in which few outer and inner petals were produced during the early flowering period, and the number of petals was relatively stable throughout the flowering period” in both H23 and H24 lines (Fig. 3). As examples of the former, outer petal number was stable, whereas inner petal number decreased week by week in H23-331 and H23-332. These lines had the greatest petal number: the maximum total number of outer and inner petals was thirty-nine in H23-331. Another example can be seen in H23-333, H24-122, and H24-131 lines: outer petal number was stable and inner petal number was low during the flowering period. In H23-333, although there were two groups that were grown under different soil conditions and regimes, they showed similar petaloidy levels in the investigation period. Thus, they were pooled and depicted in the same figure (Fig. 3).

Seasonal change of floral organ number and morphological characteristics represented by box plot. Abbreviations mean normal petals (Pt), outer petals developed close to normal petals (OPt), inner petals developed between outer petals and pistil (IPt), stamens and stamen-like organs (S). Numbers of flowers and plants investigated are also shown in parentheses under the sampling date.
In the clonal double-flowered cultivar ‘Wink Pink II’ exposed to different constant temperatures, the number of flowers did not differ between 15°C and 20°C, but it significantly decreased at 25°C (Fig. 4). There were, however, no significant differences in the rates of flowers with stamen: 16.7% at 15°C, 22.3% at 20°C, and 16.1% at 25°C. In the plants grown under different temperatures, the rate of complete petals at 15°C was the greatest, followed by the values at 25°C and 20°C (Fig. 5). In contrast to complete petals, the rate of incomplete petals without a stamen-like organ at 20°C was the greatest, followed by the values at 25°C and 15°C. The rate of stamen-like organs did not differ significantly among the treatments, while the rate of stamens was suppressed only at 25°C. Although many stamens and stamen-like organs occurred at both 15°C and 20°C, the developed positions of the stamens and stamen-like organs differed significantly between 15°C and 20°C (Table 1). They developed at sites where stamens should form originally, such as single flower at 20°C (183 out of 184 organs) (Fig. 2G–I). Compared with the normal stamen formation at 20°C, most of the stamens and stamen-like organs were produced inside petaloid-stamens at 15°C (110 out of 119 organs) (Fig. 2J–L). These additional whorls including stamen and stamen-like organ were just like secondary flowers. Although the total number of organs was in direct proportion to the total number of flowers, additional organ formation at 15°C caused a further increase of the total number of organs to the total number of flowers in comparison to the situation at 20°C (Fig. 5).

Number of flowers with or without stamen and stamen-like organ under different temperatures in double-flowered cyclamen ‘Wink Pink II’. Asterisks indicate significant difference according to Chi-square test (** P < 0.01).

Morphological variation under different temperatures in double-flowered cyclamen ‘Wink Pink II’. Different letters in rate of organs represent significant differences according to Chi-square test and residual analysis calculated from equal expected value among treatments (P < 0.05).

Number of stamens and stamen-like organs restored from petaloid-stamens or secondly formation in floral center under different temperatures in ‘Wink Pink II’.
It is recognized that the number of complete petals at 15°C was highest among treatments during the flowering period, whereas the rates of complete and incomplete petals decreased and increased, respectively, as the flowering period progressed (Fig. 6). The plants grown at 25°C also showed similar morphological alteration to those grown at 15°C. Although the rate of incomplete petals decreased at 20°C, those of stamen-like organs and stamens increased.

Time-dependent change of morphological variation under different temperatures in double-flowered cyclamen ‘Wink Pink II’. (A) Number of organs, (B) rate of organs. E: early period (1–4 w), M: middle period (5–8 w), L: later period (9–12 w). Asterisks on the right side of rate of organs indicate significant increase or decrease according to Chi-square test and residual analysis compared with previous period (* P < 0.05).
The flower stalk lengths at 20°C and 25°C were generally longer than at 15°C (Fig. 7). Most flower stalk lengths were greater in the early part of the investigation period, but decreased as the flowering period progressed. Significant differences in petal length were not recognized among treatments or weeks. Thus, the relationship between petal length and growth temperature was not clear. A difference of the petal width was also not detected among the treatments.

Time-dependent change of floral characteristics under different temperatures in double-flowered cyclamen ‘Wink Pink II’. (A) Flower stalk length, (B) petal length, and (C) petal width of normal petal. Vertical bars indicate standard errors. Means followed by different letters represent significant difference according to the Tukey-Kramer multiple comparison test of each temperature (P < 0.05).
A flower of cyclamen generally consists of five each of sepals, petals (lobes) and stamens, and a pistil (Grey-Wilson, 1988). However, in the present study, growth temperature in a greenhouse gradually increased from February to June (data not shown), and the number of petaloid-stamens decreased with the passage of time in F1 progeny that originated from double-flowered mutant (Fig. 3). At a controlled temperature, additional organ formations were recognized at a lower temperature in the clonal cyclamen cultivar ‘Wink Pink II’ (Fig. 5; Table 1). These results indicate that organ formation in the double flowers is thermo-sensitive, and the time-dependent change of environmental factors such as temperature had affected floral morphogenesis during the investigation period. It has been reported that thermo-sensitive floral homeotic gene expression alters floral morphogenesis in A. thaliana and Antirrhinum (Bowman et al., 1989; Schwarz-Sommer et al., 1992; Zachgo et al., 1995). Similarly, low and high temperatures affect the expression of C-class genes in tomato and cucumber, respectively (Kater et al., 2001; Lozano et al., 1998). Moreover, double-flowered lily cultivar ‘Elodie’ shows variation in the organ morphology of whorl 3 depending on the C-gene expression level (Akita et al., 2011). A C-class gene suppresses WUSCHEL regulating stem cell maintenance in shoot apical meristems (Lenhard et al., 2001; Lohmann et al., 2001; Nishijima, 2012; Sun et al., 2009). In a previous study, petaloid-stamen-type double-flowered cyclamen showed reduction of C-class gene expression, and it had a larger number of petaloid-organs than single flowers and other types of double flowers (Mizunoe et al., 2015). From these results, there is a possibility that a low temperature induces (or a high temperature suppresses) petaloidy and additional organ formation in double-flowered cyclamen with petaloid-stamen by reduction (or expansion) of floral homeotic gene (C-class gene) expression.
Alteration of floral morphology corresponding to the genetic background has been recognized in other flowering species. In carrot (Daucus carota), hybrid lines exhibit higher petaloidy than inbred ones (Eisa and Wallace, 1969). The temperature required for complete restoration of anthers differs among three male-sterile cultivars of Asiatic lily (Sato and Miyoshi, 2006). In double-flowered cyclamen lines, H23-333, H24-122, and H24-131 showed relatively stable organ number during anthesis (Fig. 3). In H24-122 and H24-131 lines, there was a possibility that petal number had already decreased at the beginning of the investigation because the investigation period of these groups was later than for the first group. Nevertheless, five plants in H23-333 investigated from the early flowering period also showed a stable petal number similar to H24-122 and H24-131 (Fig. 3). Therefore, it is proposed that the genetic difference between plants greatly influenced petaloidy of stamen in double-flowered cyclamen.
The number of flowers at 25°C was significantly lower than the levels at 15°C and 20°C in ‘Wink Pink II’ (Fig. 4), namely, less than half. It is implied that the high temperature, 25°C, is inappropriate for cyclamen growth. At this temperature, the rates of complete petals and stamens were lower, although that of incomplete petals was higher, than at 15°C (Fig. 5). Consequently, beneficial result was not obtained at 25°C for double-flower production. In contrast to a higher temperature, the rate of complete petals was highest at 15°C, while that of incomplete petals was lower than at 20°C and 25°C. Thus, it is indicated that a low temperature is appropriate for the stable production of double flowers. As for stamen and stamen-like organ, the constant temperature treatments for the clones showed different results between 15°C and 20°C (Table 1). At both temperatures, stamen and stamen-like organ were formed at similar levels, but they developed at different positions: the stamen and stamen-like organ formed at an inner position of petaloid-stamens at 15°C (Fig. 2G–I), although the organs were restored from the petaloid-stamen at 20°C (Fig. 2J–L). Garrod and Harris (1974) reported that a continuous low temperature or low night temperature induces the formation of additional petals in the center of flowers in carnation (Dianthus). By contrast, localized high-temperature treatment for the shoot tip increases the number of petals arising directly from the receptacle. As for each organogenesis, it was proposed that different temperatures influenced different growth substances in carnation. In the petaloid-stamen type of double-flowered cyclamen, it is considered that a higher temperature plays a role as a switch to change specification from undifferentiated female organ primordia to male organs. On the other hand, localized temperature-dependent change of floral morphology at a lower temperature is a qualitative alteration because additional organ primordia were formed anew. These two reactions responding to different temperatures may be caused by different mechanisms.
In clonal double-flowered cyclamen ‘Wink Pink II’, morphological organ characteristics differed depending on the flowering time and each plant, even though growth temperature was maintained constantly throughout the flowering period (Fig. 6). It is suggested that growth temperature was not a defining exogenous factor, but other factors are also involved in floral organogenesis.
There are close relationships between environmental factors including temperature and physiological condition, especially phytohormones. Additional petals induced by a low temperature can also be promoted by the application of GA3 and IAA in carnation (Garrod and Harris, 1974). In contrast, GA3 induces the development of normal stamens and increases the number of floral organs in L. esculentum, and the effect shows the same results as treatment with a low temperature (Sawhney, 1983; Sawhney and Greyson, 1973). In other cases, reductions of IAA and ABA concentrations are associated with the restoration of male fertility in stamenless-2 mutant of L. esculentum, which are also regulated by low temperature (Singh and Sawhney, 1998; Singh et al., 1992). Phytohormones are synthesized in various organs according to the environmental conditions to which the plant is subjected (Berleth and Sachs, 2001; Hedden and Thomas, 2012; Sakakibara, 2006). Cloned plants have the same genetic background, growth pattern, and responses to environmental conditions, but it is unlikely that the number of vegetative organs (e.g. shoot, leaf, and root) is also the same among clones. Therefore, it is assumed that variations of organ number cause quantitative differences of phytohormone synthesis in the whole plant, and the difference of delicate physiological conditions produces the individual difference of floral morphology, even in clonal double-flowered cyclamen.
This study demonstrates that a constant high temperature induces stamen formation, while the difference between day and night temperatures (DIF) is also known to be a very important factor for plant growth. There are many reports describing that thermoperiodic alteration regulates plant growth through the activation of phytohormones. For example, positive or zero DIF induces a quantitative increase of GA and/or IAA biosynthesis compared with that at a constant temperature with the same average daily temperature, and the synthesized GA and/or IAA cause stem elongations in Campanula isophylla (Jensen et al., 1996), Begonia × hiemalis (Myster et al., 1997), and Arabidopsis (Gray et al., 1998; Jouve et al., 1999; Thingnaes et al., 2003). In this study, not just stamen restoration but also significant stem elongation occurred at 20°C (Fig. 7). From the above results, synthesized phytohormones such as GA and IAA may be involved in stamen development of double-flowered cyclamen.
When we started investigation of temperature treatment, most of the flowers had complete petaloid-stamen. By contrast, many double flowers that developed under a constant temperature had an incomplete petal or staminate organ at the later period (Fig. 6). Aging can affect physiological conditions of the plant. In Alstroemeria hybrida, leaf age is related to gibberellin concentration in leaves (Kappers et al., 1997). Cistus clusii shows age-related increases in ABA levels that are affected by plant size and maturity (Oñate and Munné-Bosch, 2008). Similarly, it is suspected that nutritional conditions may have changed gradually in the later period because additional fertilizer was not applied during the investigation period in this study. Nitrogen is involved in phytohormone synthesis (Kiba et al., 2011), and leaf and root developments (Chiu et al., 2004; Walch-Liu et al., 2006). In future study, the effects of these factors should be considered to shed more light on floral morphogenesis in cyclamen.
In conclusion, floral organ number and morphology in petaloid-stamen of double-flowered cyclamen change over time, but the degree of alteration differs depending on the genetic background. Furthermore, it is indicated that a lower temperature causes additional organ formation including stamens in the center of flowers, and a higher temperature within the range appropriate for growth induces restoration of the stamen. These findings should contribute to the stable production of double-flowered cyclamen with petaloid-stamen. It is considered that double flowers that form a stamen by lower and higher temperatures or by seasonal change in the later period can be used for the production of double-flowered cyclamen as a pollen donor. However, the temperature is not the only factor regulating floral morphology in double flowers with petaloid-stamen. Further research concerning the effects of variable temperature and phytohormones on stamen formation in this type of double flower will be required to resolve the remaining issues.