2015 Volume 84 Issue 4 Pages 355-364
2015 Volume 84 Issue 4 Pages 355-364
Reciprocal crosses between nine evergreen azalea species and Rhododendron uwaense were performed to clarify the pre- and post-fertilization barriers in this interspecific hybridization for the purpose of obtaining fragrant evergreen azaleas. Unilateral incompatibility appeared in this hybridization. When evergreen azalea species were used as a seed parent, many pollen tubes stopped elongating in a style and no seed could be obtained. The reverse crosses exhibited inhibition of pollen tube penetration into ovules, chlorophyll deficiency in cotyledons, and death of young seedlings in some cross combinations. These pre- and post-fertilization barriers reduced hybridization but did not arrest it completely. As a result, many putative hybrid seedlings could be obtained. RAPD analysis revealed that 10 of the putative hybrid seedlings examined (two vigorous plants selected from respective crosses of R. uwaense #1 × five evergreen azalea species) possessed specific bands derived from both parents. The plastid DNA (ptDNA) of these seedlings was inherited maternally except for seedlings of R. uwaense #1 × R. yedoense var. poukhanense, and the ptDNA of hybrid ones obtained from R. uwaense #1 × R. yedoense var. poukhanense was inherited paternally.
Evergreen azaleas are important ornamental plants in Japan and are used in gardens, street plantings, and containers (Kunishige, 2002). A few species offer fragrance: Rhododendron mucronatum (Blume) G. Don, R. yedoense Maxim. var. poukhanense (H. Lév.) Nakai, and R. macrosepalum Maxim. have a delicate scent, the last like red clover (Galle, 1987). In cross breeding for obtaining high fragrance, North American azaleas [e.g., R. arborescence (Pursh) Torr., R. atlanticum (Ashe) Rehder, and R. viscosum (L.) Torr.] are often used as donor parents (Akabane et al., 1971; Kobayashi et al., 2008). Evergreen and North American azaleas differ from each other in subgenus: the former belong to subgenus Tsutsusi section Tsutsusi and the latter belong to subgenus Pentanthera section Pentanthera. In inter-subgeneric crosses between these two, there are pre- and post-fertilization barriers, so obtaining a hybrid is not easy (Akabane et al., 1971; Eeckhaut et al., 2003). There are some reports on the production of healthy seedlings from this cross combination, but no really worthwhile plant for commercial use has been obtained (Jaynes, 1976; Kehr, 1966; Pryor, 1973).
Rhododendron uwaense H. Hara & T. Yamanaka, belonging to subgenus Azaleastrum section Azaleastrum, has a woody floral scent. The main scent compounds are nerolidol, α-farnesene, methyl anisate, and methyl cinnamate (Ikeda and Oyama-Okubo, 2008). Section Azaleastrum is an evergreen shrub and has the novel feature that a 1–2-flowered axillary inflorescence is formed in the uppermost 1–4 leaves (Davidian, 1992). These are interesting characteristics possessed by a donor parent for evergreen azalea breeding. For these reasons, it is probable that R. uwaense is a species worthy of becoming breeding material for fragrant and multiflorous evergreen azaleas. However, the crossability between evergreen azaleas and R. uwaense is unclear because R. uwaense is a comparatively new species, registered in 1984 (Hara and Yamanaka, 1984), and its intra- and inter-specific crosses have not yet been reported. A noteworthy fact is that R. uwaense is distributed in a limited area of the western part of Shikoku, one of the four main islands of Japan, and has decreased in number to a critical level. This species is now classified as “Endangered (IB)” in the Red Data Book of Japan. Interspecific hybridization is one useful method to create new cultivars with novel characteristics. Using R. uwaense as the breeding material, we need to indicate the source and to treat the plants carefully. In addition, we have to conserve this rare species from the point of view of the genetic resources and biodiversity.
Interspecific pollinations within the Rhododendron genus are usually only successful when the parents belong to the same subgenus (Eeckhaust et al., 2003), and cross incompatibility is commonly recognized in wide crosses between subgenera (Kehr, 1977). From cladistic analysis using molecular data, the subgenus Azaleastrum was shown to be polyphyletic, and section Azaleastrum species showed a sister group relationship to evergreen azalea species (Kurashige et al., 2001). Goetsch et al. (2005) proposed that the genus Rhododendron was subdivided into five subgenera, and the subgenus Tsutsusi was reduced to a section of the subgenus Azaleastrum. So far, we have investigated pre- and post-fertilization barriers and have evaluated the potential for inter-subgeneric crosses of evergreen azalea species × R. japonicum (A. gray) J. V. Suringar f. flavum Nakai, belonging to the subgenus Pentanthera (Okamoto and Suto, 2004; Ureshino et al., 2000), and Kurume azalea (R. × obtusum Planch. cultivars) × scaly rhododendrons, belonging to the subgenus Rhododendron (Okamoto and Ureshino, 2010). In the present study, reciprocal crosses between evergreen azalea species and R. uwaense were undertaken to clarify the pre- and post-fertilization barriers in this hybridization.
From 2009 to 2012, nine species of wild evergreen azalea and two plants of R. uwaense were used (Table 1), and reciprocal crosses were conducted under unheated greenhouse conditions. Concerning nine evergreen azalea species, one individual was used for the respective species. All plants were five to 21 years old, derived from rooted cuttings and grown at the National Agriculture and Food Research Organization (NARO) Kyushu Okinawa Agricultural Research Center, Kurume, Fukuoka Prefecture, Japan. The function of both male and female gametophytes was checked by self-pollination, and all plants produced germinable seeds.

Rhododendron species used in this study.
Flower buds were emasculated at the beginning of anthesis. Anthers were collected from the flower buds at the beginning of anthesis. At a stigma covered with exudates, three flowers for respective crosses were pollinated with fresh and one-year-old pollen stored at 5°C.
Capsules were collected in November, and the number of mature seeds per capsule was counted. In late February, approximately 200 seeds were sown on seedling beds consisting of Kanuma volcanic sand covered with a layer of sphagnum moss. The seedling beds were placed in an unheated greenhouse that reduced the photosynthetic photon flux density (PPFD) by 28% and under intermittent mist. Germinating seeds were counted once every two weeks from April to May, and the color of cotyledons was noted. In June, the seedling beds were transferred to another greenhouse where the PPFD was reduced by 17%. The number of viable seedlings was counted nine months after sowing. For the hybridity, putative hybrid seedlings were distinguished from non-hybrid ones by the color of unexpanded leaves in spring. The unexpanded leaves of the evergreen azalea species and R. uwaense were yellow green (the Royal Horticultural Society (RHS) colour chart No. 144C) and greyed red (RHS colour chart No. 184B), respectively, and those of the putative hybrids were olive green (RHS colour chart No. 152C). In this study, R. eriocarpum (Hayata) Nakai × R. uwaense was excluded because contamination by self-pollination could not be prevented.
Microscopic observation before and after fertilizationPollination utilized the same procedure as described above, and six flowers were pollinated for respective crosses between evergreen azalea species and R. uwaense #1 in 2011. The style length of the parental plants was measured for 10 pistils. The style length ratio (SLR) was calculated as follows: pollen parent/seed parent (male/female) style lengths. Ten days after pollination, three pistils were collected from respective seed parents, fixed in FAA (1 formalin:1 acetic acid:18 70% ethanol, volume) for 24 h, after which they were transferred to 70% ethanol and stored at room temperature. The fixed pistils were softened by soaking in 1 N NaOH at 38°C for 16 h, washed in distilled water, and stained with 0.1% aniline blue in 0.1 N K3PO4 for a minimum of 3 h. After the styles and ovaries were separated, the latter were cut lengthwise in half and carpels were picked out from each ovary using tweezers. The styles and carpels were placed on separate microscope slides and subjected to gentle pressure on a coverslip. The number of pollen tubes in the lower style and the number of ovules penetrated by pollen tubes were counted under a biological microscope (ECLIPSE E400; Nikon Corp., Tokyo, Japan) with a high-pressure mercury vapor lamp and combined ultraviolet excitation filter (EX365/10, DM400, BA400; Nikon Corp., Tokyo, Japan).
Twenty days after pollination, the remaining three pistils were collected from the respective seed parents and fixed with FAA. After the styles and ovaries had been separated, the latter were dehydrated using an ethanol-butanol series and embedded in paraffin blocks. Sections were cut to 12 μm thickness with a rotary microtome (PR-50; Yamato Kohki Industrial Co., Ltd., Saitama, Japan) and were stained with 1% fuchsin acid and 0.2% fast green. The structure of the embryo sacs was observed using the above microscope, and the number of ovules having a zygote with free nuclear division of endosperm after fertilization (fertilized ovules) and the number of ovules penetrated by pollen tubes not leading to fertilization (unfertilized ovules) were counted (Fig. 1).

Longitudinal sections of ovules, Rhododendron uwaense #1 × R. kaempferi. A, ovule having a zygote with free nuclear division of endosperm after fertilization; B, ovule penetrated by a pollen tube not leading to fertilization. e, endosperm; m, micropyle; p, pollen tube; z, zygote. Scale bar = 100 μm.
In 2011, 10 putative hybrid seedlings (two vigorous plants selected from respective crosses of R. uwaense #1 × five evergreen azalea species, R. eriocarpum (Hayata) Nakai, R. kaempferi Planch., R. macrosepalum, R. ripense Makino, and R. yedoense var. poukhanense) and their parents were used. Total genomic DNA was extracted from approximately 70 mg of frozen leaves by a modified CTAB method (Kobayashi et al., 1998). Random amplified polymorphic DNA (RAPD) analysis was conducted with CMN-A02 (5'-GCCAGCTGTACG-3') and CMN-B27 (5'-CGCAGCCGAGAT-3') common primers (BEX, Co., Ltd., Tokyo, Japan) to confirm the hybridity of the seedlings. Amplifications were performed in 25 μL of reaction solution containing 20 ng of genomic DNA, 0.5 μM primers, 0.1 mM dNTPs, 2 mM MgCl2, 1× the original reaction buffer, and 0.5 units Taq DNA polymerase (La Roche Ltd., Basel, Switzerland). DNA was amplified with a DNA thermal cycler (TP240; Takara Bio Inc., Shiga, Japan) as follows: 1 cycle of 30 sec at 90°C; 45 cycles of 30 sec at 94°C, 2 min at 37°C, and 3 min at 72°C; and 1 cycle of 7 min at 72°C. The amplified products were electrophoresed in 1.5% agarose gels (Sigma-Aldrich Co. LLC, St. Louis, MO, USA) with 1 × TBE. The gel was stained with an ethidium bromide solution and observed under ultraviolet illumination. To clarify the plastid DNA (ptDNA) inheritance of the seedlings, polymerase chain reaction single-strand conformation polymorphism (PCR-SSCP) analysis at the chloroplast trnL–trnF and trnG–trnM intergeneric regions was conducted as described in a previous report (Itabashi et al., 2008). For the investigation of ptDNA inheritance, molecular marker techniques such as RFLP and PCR-RFLP have been used (Chat et al., 1999; Yao et al., 1994). These methods are very powerful tools to detect polymorphism among species. However, to identify polymorphism with these methods, mutation in a restriction site is required. Therefore, it is difficult to detect polymorphism among closely related species. As a more sensitive polymorphic marker, the PCR-SSCP method has been used to detect polymorphism within species or among some closely related species and to investigate the inheritance pattern of ptDNA (Chen et al., 2002; Shiraishi et al., 2001). Furthermore, the PCR-SSCP method has been used to study chloroplast DNA inheritance in azaleas (Itabashi et al., 2008; Ureshino et al., 2006, 2010). Therefore, we used this method to clarify ptDNA inheritance.
Capsules set only when R. uwaense was used as a seed parent (Table 2). In crosses using R. uwaense as the seed parent, the number of mature seeds per capsule varied from 73 to 313, and the control cross (R. uwaense #2 × R. uwaense #1) had 518 (Table 2). The germination rate varied from 40.0% to 83.0%, with 53.0% in the control (Table 2). The cotyledons were green, pale green, and white. The albino rate varied from 0% to 28.3%, and 10 out of 16 crosses had a rate of less than 5% (Table 3). Albino seedlings were not found in the control. The pale green and white seedlings wilted until the formation of the first true leaf. The percentage of viable seedlings/germinating seedlings excluding albinos (survival rate) varied from 22.8% to 67.5%, with 84.9% in the control (Table 3). From these data, we calculated the estimated number of viable seedlings per pollinated flower for respective crosses. This value ranged from 14.3 to 68.3, with 233.1 in the control (Table 3), and showed variation among the pollen parents (Table 4); more viable seedlings were produced when R. uwaense was pollinated by R. eriocarpum than by R. kiusianum Makino, R. serpyllifolium Miq., and R. yedoense var. poukhanense on the basis of Tukey’s HSD test. Most of the green seedlings were putative hybrids that had olive green unexpanded leaves and were healthy two years after sowing. The growth of the putative hybrid seedlings compared favorably with that of control ones (Fig. 2).

Capsule and seed yields, and germination rate in crosses between evergreen azalea species and Rhododendron uwaense.
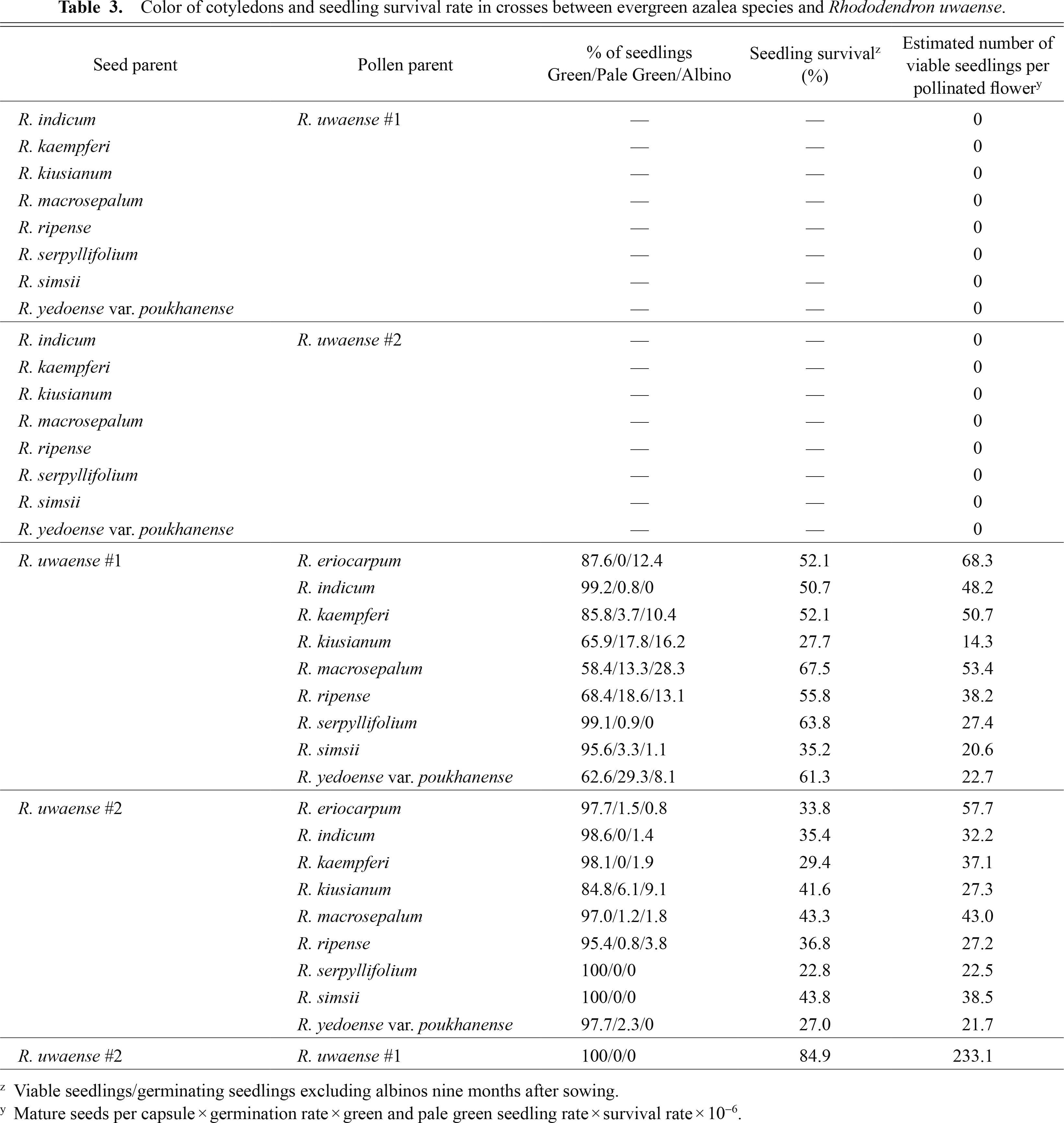
Color of cotyledons and seedling survival rate in crosses between evergreen azalea species and Rhododendron uwaense.

Analysis of variance on the estimated number of viable seedlings per pollinated flower in Rhododendron uwaense × nine evergreen azalea species.

Putative hybrid seedlings of R. uwaense #1 × R. macrosepalum three years after sowing.
The male/female SLR of R. uwaense #1 × evergreen azalea species varied from 0.71 to 2.65, and that of reverse crosses varied from 0.38 to 1.41, with 0.88 in the control (Table 5). In the crosses of R. uwaense #1 × evergreen azalea species, the number of pollen tubes in the lower style exceeded 200 (Table 5). The percentage of ovules penetrated by pollen tubes varied from 26.3% to 44.3% (Table 5). In the reverse crosses, many pollen tubes stopped elongating at the mid-portion of the style and several abnormalities of arrested pollen tube tips were detected, including mainly tapered and swollen types (Fig. 3A, B). In addition, spiraling tubes without defined callose plugs were found occasionally (Fig. 3C). As a result, there were fewer than 10 pollen tubes in the lower style, and the percentage of ovules penetrated by pollen tubes varied from 0% to 2.4% (Table 5). In the control, there were 400 to 600 pollen tubes in the lower style, and 86.1% of ovules were penetrated by pollen tubes (Table 5). In the crosses of R. uwaense #1 × evergreen azalea species, the percentage of fertilized ovules per ovule penetrated by pollen tubes varied from 71.5% to 84.3%, with 82.8% in the control (Table 5). For the reverse crosses, microscopic observation was not performed because R. uwaense’s tubes seldom penetrated into the ovules of evergreen azalea species.

Style length ratio, number of pollen tubes in a style, percentage of ovules penetrated by pollen tubes, and fertilized ovule rate in crosses between evergreen azalea species and Rhododendron uwaense.

Fluorescence microscopy of incompatible pollen tube behavior, Rhododendron kaempferi × R. uwaense #1. A, tapered tube tip (arrow); B, swollen tube tip; C, spiraling tube without defined callose plugs. Scale bar = 100 μm.
RAPD analysis with CMN-A02 and CMN-B27 primers indicated that all 10 seedlings examined (R. uwaense #1 × evergreen azalea species) were of hybrid origin, having specific bands derived from both parents, that is, the seedlings of R. uwaense #1 × R. eriocarpum, R. kaempferi, R. macrosepalum, and R. ripense were confirmed as hybrids by CMN-A02 (Fig. 4). Similarly, those of R. uwaense #1 × R. eriocarpum, R. kaempferi, and R. yedoense var. poukhanense were confirmed as hybrids by CMN-B27 (Fig. 5). A difference in phenotype was detected between evergreen azalea species and R. uwaense #1 by PCR-SSCP analysis in the chloroplast trnL–trnF and trnG–trnM intergeneric regions (Fig. 6). All seedlings examined had the haplotype of R. uwaense #1, except for two seedlings of R. uwaense #1 × R. yedoense var. poukhanense, and the seedlings of which R. yedoense var. poukhanense was the pollen parent had the haplotype of R. yedoense var. poukhanense (Fig. 6). Therefore, ptDNA inheritance of R. uwaense #1 × evergreen azalea species was maternal, except for the cross of R. uwaense #1 × R. yedoense var. poukhanense, and ptDNA of the hybrid seedlings from R. uwaense #1 × R. yedoense var. poukhanense was inherited paternally.

Amplified polymorphisms of putative hybrid seedlings obtained from Rhododendron uwaense #1 × evergreen azalea species using primer CMN-A02. Arrows indicate the specific band for R. uwaense. Arrowheads indicate the specific band for each evergreen azalea species. Lane 1, R. uwaense #1; Lanes 2 and 3, seedlings from R. uwaense #1 × R. ripense; Lane 4, R. ripense; Lane 5, R. uwaense #1; Lanes 6 and 7, seedlings from R. uwaense #1 × R. macrosepalum; Lane 8, R. macrosepalum; Lane 9, R. uwaense #1; Lanes 10 and 11, seedlings from R. uwaense #1 × R. kaempferi; Lane 12, R. kaempferi; Lane 13, R. uwaense #1; Lanes 14 and 15, seedlings from R. uwaense #1 × R. eriocarpum; Lanes 16 and 17, self-pollinated seedlings of R. eriocarpum; Lane 18, R. eriocarpum; Lane M, 100 bp ladder DNA size markers.

Amplified polymorphisms of putative hybrid seedlings obtained from Rhododendron uwaense #1 × evergreen azalea species using primer CMN-B27. Arrows indicate the specific band for R. uwaense. Arrowheads indicate the specific band for each evergreen azalea species. Lane 1, R. uwaense #1; Lanes 2 and 3, seedlings from R. uwaense #1 × R. ripense; Lane 4, R. ripense; Lane 5, R. uwaense #1; Lanes 6 and 7, seedlings from R. uwaense #1 × R. macrosepalum; Lane 8, R. macrosepalum; Lane 9, R. uwaense #1; Lanes 10 and 11, seedlings from R. uwaense #1 × R. yedoense var. poukhanense; Lane 12, R. yedoense var. poukhanense; Lane 13, R. uwaense #1; Lanes 14 and 15, seedlings from R. uwaense #1 × R. kaempferi; Lane 16, R. kaempferi; Lane 17, R. uwaense #1; Lanes 18 and 19, seedlings from R. uwaense #1 × R. eriocarpum; Lanes 20 and 21, self-pollinated seedlings of R. eriocarpum; Lane 22, R. eriocarpum; Lane M, 100 bp ladder DNA size markers.

PCR-SSCP banding patterns of PCR products amplified for the chloroplast trnL-trnF and trnG-trnM intergeneric regions in hybrid seedlings obtained from Rhododendron uwaense #1 × evergreen azalea species. E, R. eriocarpum; K, R. kaempferi; M, R. macrosepalum; R, R. ripense; U, R. uwaense #1; Y, R. yedoense var. poukhanense.
The genus Rhododendron is divided into eight subgenera (Chamberlain et al., 1996). Tsutsusi (evergreen azaleas except trifoliate azaleas), Pentanthera (deciduous azaleas), Rhododendron (scaly rhododendrons and Malesian rhododendrons), and Hymenanthes (non-scaly rhododendrons) are important subgenera for horticultural use, and breeders have attempted crosses among these four classes (Kehr, 1977). Subgenus Azaleastrum species, however, were rarely used in interspecific hybridization.
In this study, unilateral incompatibility appeared in the hybridization between evergreen azalea species and R. uwaense. Most pollen tubes of R. uwaense did not reach the ovaries of the evergreen azalea species. Kho and Baër (1973) demonstrated that unilateral interspecific incompatibility was observed in Rhododendron crosses, which was attributed to the inability of pollen of a short-styled species to traverse the style of a longer-styled species. Williams and Rouse (1988) reported that successful interspecific fertilization was closely related to male/female SLR in interspecific crosses among species belonging to subgenus Rhododendron section Vireya. They determined that the crosses with a male/female SLR of less than 0.2 or greater than 6.0 were all unsuccessful and that the probability of success increased as the SLR approached 1.0. In our unsuccessful crosses, the range of SLR was 0.38 to 1.41, implying that SLR did not restrict R. uwaense’s pollen tube growth into evergreen azalea pistils.
Williams et al. (1982) reported that incompatible pollen tubes exhibited errors of tip growth and callose deposition anomalies after foreign pollination in the genus Rhododendron. In the crosses of evergreen azalea species × R. uwaense, many pollen tubes disappeared rapidly at the mid-portion of the style, and abnormal morphology of tube tips was observed. Hence, we consider that pollen tube growth is arrested by errors of tube tip growth. Therefore, crosses of evergreen azalea species × R. uwaense suffer from a pre-fertilization barrier and yield no seed.
Using R. uwaense as the seed parent, the arrest of pollen tube growth was not found in the pistil and many pollen tubes could reach the ovaries. The percentage of ovules penetrated by pollen tubes was relatively low compared with that of the control, indicating that the inhibition of pollen tubes penetrating into the ovules is one cause of the pre-fertilization barrier. Kaul et al. (1986) determined that incompatible interspecific crosses exhibited seed failure until 15 days after pollination because of failure of zygote and/or endosperm development. In our crosses, many ovules penetrated by pollen tubes developed normally 20 days after pollination, indicating that seed failure did not cause the post-fertilization barrier. The number of mature seeds per capsule was relatively low compared with that of the control, and it seems that this mainly arises from the inhibition of pollen tubes penetrating into ovules. Okamoto and Suto (2004) reported that the lack of germination was one cause of a post-fertilization barrier in crosses of evergreen azalea species × R. japonicum. In this study, the germination rate was, on the whole, at a high or similar level compared with that in the control, indicating that no particular incompatibility appears in this location. The white color of the cotyledons was the result of hybrid chlorophyll deficiency, and caused by interspecific incompatibility between the plastid genome and the nuclear genome, that is, plastome-genome incompatibility (Ureshino et al., 1999). Albino seedlings were detected in many crosses, but the albino rate was generally low. Therefore, the chlorophyll defect is one cause of the post-fertilization barrier, although its effect may be limited. All crosses had a survival rate lower than that of the control, indicating that death of young seedlings (hybrid inviability) is one cause of the post-fertilization barrier. Thus, the crosses of R. uwaense × evergreen azalea species have pre- and post-fertilization barriers; each barrier reduces hybridization, but does not arrest it completely, and healthy putative hybrid seedlings can be obtained. Amongst these crosses, the cross of R. uwaense × R. eriocarpum exhibited a greater estimated number of viable seedlings per pollinated flower than those of R. uwaense × R. kiusianum, R. serpyllifolium, and R. yedoense var. poukhanense. In this study, one individual was used for the respective evergreen azalea species. We consider that it is necessary to use more plants for the respective species in order to be able to discuss the differences in this trait among species.
It is known that the mode of ptDNA inheritance is biparental in Rhododendron (Corriveau and Coleman, 1988; Miyamura et al., 1987; Nagata et al., 1999). Ureshino et al. (1999) reported that ptDNA of green seedlings obtained from evergreen azaleas × R. japonicum was derived from R. japonicum (paternal inheritance) and that of albino ones was inherited maternally. Okamoto and Ureshino (2010) reported that the ptDNA of vigorous seedlings obtained from evergreen azaleas × scaly rhododendron, R. keiskeii Miq., was derived from R. keiskei (paternal inheritance). These phenomena imply that plastome-genome incompatibility occurs between a plastid genome from evergreen azaleas and a nuclear genome from foreign subgenera. In this study, the hybrid seedlings obtained from R. uwaense × evergreen azalea species had ptDNA derived from R. uwaense (maternal inheritance) except for R. uwaense #1 × R. yedoense var. poukhanense. This ptDNA inheritance is similar to the above phenomenon. The ptDNA inheritance detected in the cross of R. uwaense #1 × R. yedoense var. poukhanense is an exceptional case because plastome-genome incompatibility between a plastid genome from R. yedoense var. poukhanense and a nuclear genome from R. uwaense is resolved. Ureshino and Miyajima (2002) reported that a triploid green seedling with 2x and 1x nuclear genomes from evergreen azalea and R. japonicum, respectively, had ptDNA derived from the evergreen azalea. In addition, plastome-genome incompatibility was broken by a relative increase of the gene from evergreen azaleas in the nuclear gene of hybrid seedlings (Ureshino et al., 2010). The mechanism of ptDNA inheritance detected in the cross of R. uwaense #1 × R. yedoense var. poukhanense may become clear when ploidy and isozyme analyses are performed.
We have carried out inter-subgeneric crosses of evergreen azaleas with deciduous azalea, R. japonicum (Okamoto and Suto, 2004), and scaly rhododendrons, R. keiskei and R. mucronalutum Turcz. var. ciliatum Nakai (Okamoto and Ureshino, 2010). These hybridizations had several causes of pre- and post-fertilization barriers, and the production of viable seedlings per pollinated flower was generally low or absent. In the crosses of R. uwaense × evergreen azalea species, many viable seedlings were obtained from one pollinated flower, despite pre- and post-fertilization barriers being detected. The difficulty of creating interspecific hybrids increases along with the phylogenetic distance between the parents (Sharma, 1995). Section Azaleastrum species were shown to be nested within evergreen azalea species based on molecular phylogeny with matK and trnK (Kurashige et al., 2001) and RPB2-I (Goetsch et al., 2005) sequence analysis. Our findings in this study are consistent with their phylogenetic data. A similar case was found in intergeneric hybridization between Menziesia multiflora var. purpurea (Makino) Ohwi and evergreen azalea species (Kita et al., 2005). Menziesia species were placed in the same clade of evergreen azalea species based on molecular phylogenetic data (Kron, 1997; Kurashige et al., 2001). These results demonstrate that molecular information may bring about a better understanding of the rationale for the degree of reproductive isolation observed.
In this study, molecular screening for true hybrids was not performed for all seedlings because this research focused on pre- and post-fertilization barriers between evergreen azalea species and R. uwaense. Further research will focus on hybrid detection using molecular techniques and the scents of hybrid plants.