2016 Volume 85 Issue 2 Pages 161-168
2016 Volume 85 Issue 2 Pages 161-168
An unidentified compound was detected in sweet pea (Lathyrus odoratus L. ʻDianaʼ) petals by HPLC analysis using a cation-exchange column for soluble carbohydrate analysis. This compound was identified as 2-cyanoethyl-isoxazolin-5-one (2-CEIX) using 1H-NMR, 13C-NMR, and CI-MS and ESIMS. 2-CEIX was detected in the petals, leaves and stem. Amino acid and other nitrogenous compound contents in these organs were compared with 2-CEIX. The content of asparagine was highest, followed by 2-CEIX in the petals, and 2-CEIX was highest among nitrogenous compounds in the stem and leaves. The 2-CEIX content in the petal decreased during flower opening, but those in the petals and the other floral parts increased during senescence regardless of sucrose treatment. These trends differed from those of monosaccharides, sucrose and cyclitols. Thus, the role of 2-CEIX appears to differ from those of soluble carbohydrates. 2-CEIX was not detected in phloem and xylem saps. The results suggest that 2-CEIX is a major nitrogenous compound of low molecular weight and is likely to be produced in situ in various organs in sweet pea.
Sweet peas are suitable for cut flowers because of their wide range of colors and good fragrance. The flowers of sweet pea are highly sensitive to ethylene, and the vase life of their cut flowers is very short (Ichimura and Hiraya, 1999; Kebenei et al., 2003; Mor et al., 1984; Sexton et al., 1995). Treatment with sucrose supplemented with 8-hydroxyquinoline sulfate (8-HQS), an antimicrobial compound, promotes flower opening, and extends vase life in cut sweet pea flowers (Ichimura and Hiraya, 1999; Ichimura and Suto, 1999). These positive effects have been attributed to increase in soluble carbohydrate contents and delay in climacteric-like increase in ethylene production (Ichimura and Suto, 1999). For flower opening, large amounts of soluble carbohydrates are required as substrates for respiration, osmotica and synthetic materials. Indeed, the contents of soluble carbohydrates, including glucose and sucrose, in the petals increase during flower opening in sweet pea (Ichimura et al., 1999). Thus, soluble carbohydrates are important for the flower opening and vase life of cut sweet peas.
Previously, Ichimura et al. (1999) reported the presence of glucose, fructose, sucrose, myo-inositol and l-bornesitol as soluble carbohydrate constituents in the petals of sweet pea. Furthermore, a compound was detected by HPLC analysis using a column for carbohydrate analysis. The compound corresponding to the peak was isolated, and subjected to structural analysis, but could not be identified in the previous study (Ichimura et al., 1999).
In the present study, we successfully conducted the identification of this compound as 2-cyanoethyl-isoxazolin-5-one (2-CEIX), a nitrogenous compound. Although 2-CEIX was previously identified in sweet pea (Ikegami et al., 1984; Van Rompuy et al., 1974a), it remains unclear whether 2-CEIX is the major nitrogenous compound in this plant. We, thus, investigated 2-CEIX and amino acid contents in various organs in sweet peas. To clarify similarities in role between 2-CEIX and soluble carbohydrates, changes in 2-CEIX contents in the flowers during flower opening and sucrose treatment were compared with those in soluble carbohydrate contents. We also investigated compounds in phloem and xylem sap to explore the possibility that 2-CEIX is transported through phloem or xylem.
Sweet peas (Lathyrus odoratus L. ‘Diana’) were grown in a greenhouse under frost-free conditions. To determine the content of amino acids and other nitrogenous compounds, including 2-CEIX in the petals, stem and leaves, these organs were collected from flowering stems. The petals were collected from flowers within 1 day of opening. To determine 2-CEIX contents in the petals during flower bud development, petals were collected from flowers at 5 different stages: Stage 1; bud length was about 1.5 cm. Stage 2; bud length was about 2 cm. Stage 3; bud length was about 2.5 cm. Stage 4; bud length was about 3 cm. Stage 5; flower within 1 day of opening.
Isolation and identification of compoundFor isolation, 20.5 g fresh weight (FW) of petals was taken from the flowers within 1 day of opening, and extracted with 10 volumes of 80% ethanol at 75°C for 30 min, then homogenized. The homogenate was centrifuged at 3000 × g for 10 min. The resulting supernatant was evaporated in vacuo below 50°C. The concentrate was dissolved in a minimum of water, and passed through a Sep-Pak C-18 (Millipore, Billerica, MA, USA) with water. The eluate was separated using HPLC with a refractive index detector on a Shodex C18-5E (Φ10 × 300 mm; Showa Denko, Tokyo, Japan), which was eluted with water at a flow rate of 2 mL·min−1. Fractions containing peak A were finally purified on a Pb-loaded cation exchange column of Shodex Sugar SP0810 column, which was kept at 80°C, and eluted with water at a flow rate of 0.8 mL·min−1. A solid was obtained after removal of water.
The chemical structure of the purified compound was analyzed by CI-MS and ESIMS using a JMS-SX 102A instrument (Jeol, Tokyo, Japan) and 1H-NMR (270 MHz, D2O), 13C-NMR (68 MHz, D2O), 1H-1H COSY and 1H-13C COSY using a Jeol JNM-EX270 instrument.
Determination of 2-CEIX contents in petals, the other floral parts, stem, and leaves2-CEIX was extracted based on the conventional method for soluble carbohydrate extraction as reported by Norikoshi et al. (2009). Briefly, petals, the other floral parts that included stamens, pistil and calyx, stem and leaves (1 g FW) obtained from 3 plants was extracted with 80% ethanol, and purified using the Sep-Pak C18. 2-CEIX contents were measured using HPLC on a column of Shodex Sugar SP0810 (Showa Denko). The column was kept at 80°C, and eluted with water at a flow rate of 0.8 mL·min−1. The contents of 2-CEIX were calculated as described in Norikoshi et al. (2009).
Determination of amino acids and nitrogenous compoundsThe petals, stem and leaves (1 g FW each) were immersed in 10 volumes of 80% ethanol at 75°C for 30 min, then homogenized. The homogenate was centrifuged at 3000 × g for 10 min. The resulting supernatant was evaporated in vacuo below 50°C. The residue was immersed in 1% 5-sulfosalicylic acid solution. Amino acid and nitrogenous compound contents were determined using an amino acid analyzer (JLC-500/V2; Jeol) according to the manufactureʼs instruction manual.
Sucrose treatment to cut flower spikesFlower spikes, each with 3 buds, were cut from the plants when the first bud from the base of each spike was thought likely to open within the next day. The spikes were trimmed to 15 cm, and three cut spikes were placed in 50-ml Erlenmeyer flasks with 40 g·L−1 sucrose supplemented with 200 mg·L−1 8-hydroxyquinoline sulfate (8-HQS). 8-HQS solution was used as the control. The flower spikes were kept at 23°C, 70% relative humidity, and under a 12-h photoperiod with 10 μmol·m−2·s−1 photosynthetic photon flux using cool-white fluorescence lamps.
Collection of xylem sapThe sweet pea plants with length of about 50 cm were cut at ground level, and then allowed to stand for about 30 min. The sap on the cut surface was collected from five plants using a syringe. Soluble carbohydrate composition of the collected saps was analyzed by HPLC as described above.
Analysis of substances for translocation in phloemStems with two leaves were cut from sweet pea plants in the morning. The stems were re-cut to 20 cm in length, and the cut end was put into water. The stems were kept at 25°C under 250 μmol·m−2·s−1 photosynthetic photon flux using cool-white fluorescence lamps for about 1 h. Leaves were cut from the stems, and placed in 27-L acrylic chamber. Then, 14C-sodium bicarbonate (NaH14CO3, 9.25 MBq, 74 MBq·mmol−1) was dissolved in water, and lactic acid was added to the solution to evolve 14CO2. Leaves were allowed to stand for 30 min for photosynthesis, after which the cut ends were placed in 20 mM EDTA solution, and kept at 20°C in darkness for 3 h to collect phloem sap. The solution containing 14C-labelled compound was evaporated to dryness, and re-dissolved in distilled water. The 14C-labelled compound was analyzed by HPLC as described above except that a radio analyzer (Aloka, Tokyo, Japan) was equipped.
Figure 1A shows the elution profile of the ethanol extract from petals of sweet peas by HPLC analysis using a Pb-loaded cation exchange column. Besides glucose, fructose, sucrose, l-bornesitol and myo-inositol, an unidentified major peak, U was detected. Fractions containing peak U were purified to yield 17 mg of the compound.

HPLC elution profile on a Pb-loaded cation exchange column of ethanol extract from sweet pea petals (A) and chemical structure of the isolated compound from sweet pea petals (B). A. 1, sucrose; 2, glucose; 3, fructose; 4, bornesitol; 5, myo-inositol; U, 2-cyanoethyl-isoxazolin-5-one. B. Chemical structure of 2-cyanoethyl-isoxazolin-5-one.
CI-MS and ESIMS analyses gave [M + H]+ at m/z 139.0503, indicating that [M]+ of this compound is 138.0425, which was calculated for C6H6N2O2 of m/z 138.0411. Among compounds, which have been reported to be much contained in sweet pea, 2-CEIX (Kuo et al., 1982) has the coincided molecular composition (Fig. 1B), and all NMR spectra were assigned to 1H and 13C of this compound, resulting in the identification.
1H-NMR δ: 2.93 (2H, t, J = 6.3 Hz, cyanoethyl moiety H-2), 4.02 (2H, t, J = 6.3 Hz, cyanoethyl moiety H-1), 5.30 (1H, d, J = 3.7 Hz, isoxazolin-5-on moiety H-4), 8.51 (1H, d, J = 3.7 Hz, isoxazolin-5-on moiety H-3); 13C-NMR δ: 16.0 (cyanoethyl moiety C-2), 47.9 (cyanoethyl moiety C-1), 88.4 (isoxazolin-5-on moiety C-4), 118.3 (CN), 155.0 (isoxazolin-5-on moiety C-3), 170.1 (isoxazolin-5-on moiety C-5).
Contents of 2-CEIX, amino acids, and other nitrogenous compounds in petals, stem, and leavesContents of 2-CEIX were compared with those of amino acids and other nitrogenous compounds in the petals, leaves and stems. Twenty one amino acids and some nitrogenous compounds, including NH3 and urea, were detected in the petals (Table 1). Among these compounds, the asparagine content was the highest, followed by α-alanine in the petals. 2-CEIX had the third largest content in the petals. Total amino acid contents were lower in the leaves and stems than that in the petals. 2-CEIX contents were much higher than those of amino acid and other nitrogenous compounds in stem and leaves.

Contents of 2-cyanoethyl-isoxazolin-5-one, amino acids, and nitrogenous compounds in petals, stem, and leaves in sweet pea.
The 2-CEIX content was 22.4 μmol·g−1 FW at stage 1, and almost constant between stages 1 and 4, but decreased during flower opening (Fig. 2A). In contrast, monosaccharide and sucrose contents increased during flower opening whereas the cyclitol content slightly decreased.

Changes in 2-cyanoethyl-isoxazolin-5-one and soluble carbohydrate contents per fresh weight (A) and per flower (B) bases in petals during flower opening. Values are mean ± SE (n = 3). Monosaccharide (glucose and fructose), sucrose and cyclitol (myo-inositol and bornestol) contents were calculated from data reported by Ichimura et al. (1999).
The content of 2-CEIX per flower increased by about 6 times between stages 1 and 4, but decreased during flower opening (Fig. 2B). In contrast, monosaccharide and sucrose contents markedly increased during flower development and opening.
We confirmed that monosaccharide composition consisted of glucose (83%) and fructose (17%), whereas cyclitol composition consisted of bornesitol (89%) and myo-inositol (11%) at stage 5.
Changes in 2-CEIX and soluble carbohydrate contents in petals and the other floral parts in cut flower spikes during sucrose treatmentThe petals in the control and sucrose-treated flowers wilted on days 3 and 5, respectively. In the petals, monosaccharide and sucrose contents decreased with time, and sucrose treatment increased these sugar contents. In contrast, the cyclitol content was more or less constant regardless of sucrose treatment. The 2-CEIX content in the petals in the control flowers increased with time, and this increase continued by sucrose treatment (Fig. 3). Similar trends were observed in the other floral parts (Fig. 4).

Changes in 2-cyanoethyl-isoxazolin-5-one and soluble carbohydrate contents in petals after sucrose treatment. Cut flowers were treated with 200 mg·L−1 8-HQS solution (A), or a solution containing 40 g·L−1 sucrose and 200 mg·L−1 8-HQS (B). Values are mean ± SE (n = 3). Monosaccharide (glucose and fructose), sucrose and cyclitol (myo-inositol and bornestol) contents were calculated from data reported by Ichimura et al. (1998).

Changes in 2-cyanoethyl-isoxazolin-5-one and soluble carbohydrate contents in the other floral parts after sucrose treatment. Cut flowers were treated with 200 mg·L−1 8-HQS solution (A), or a solution containing 40 g·L−1 sucrose and 200 mg·L−1 8-HQS (B). Values are mean ± SE (n = 3). Monosaccharide (glucose and fructose), sucrose and cyclitol (myo-inositol and bornestol) contents were calculated from data reported by Ichimura et al. (1998).
In the xylem exudates, 2-CEIX was not detected, but only sucrose was detected by HPLC analysis (data not shown). This indicates that 2-CEIX is not transported from the roots through xylem.
To clarify whether 2-CEIX is a substance for translocation, 14C-labelled compound in the phloem exudates was investigated. 14C-Sucrose was only detected in HPLC separation using the radio analyzer (Fig. 5). This suggests that 2-CEIX is not a substance for translocation, but sucrose is a substance for translocation in sweet pea.
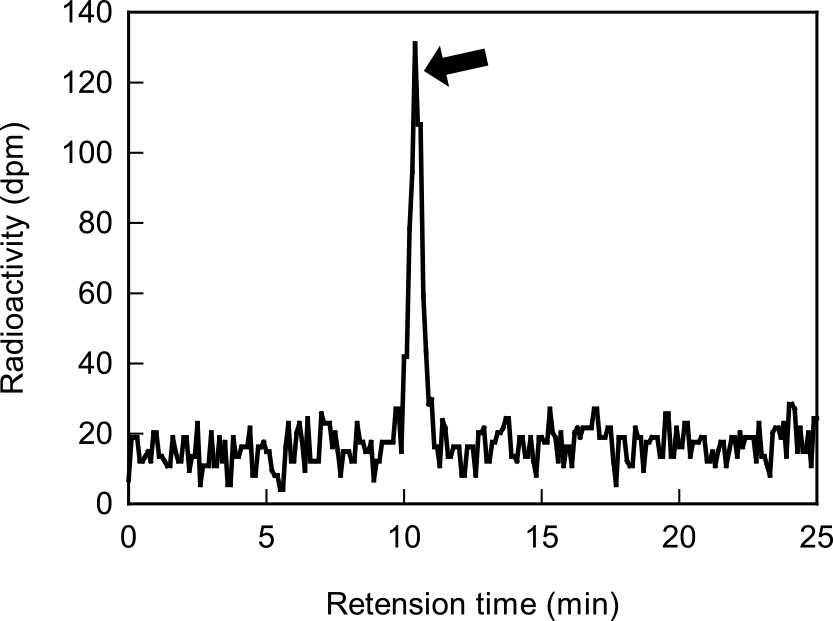
HPLC elution profile on a Pb-loaded cation exchange column of 14C-compounds detected by the radio analyzer in phloem sap obtained from sweet pea. Arrow indicates a peak of which retention time corresponds to sucrose.
In the present study, 2-CEIX was identified in sweet pea. We previously thought that this compound might be a soluble carbohydrate (Ichimura et al., 1999), because this compound is clearly separated using the HPLC column for soluble carbohydrate analysis (Fig. 1A). This may be due to its high hydrophilic and neutral property. To the best of our knowledge, 2-CEIX was identified first in sweet pea (Van Rompuy et al., 1974a), and has only been found in Lathyrus plants (Ikegami et al., 1984; Lambein et al., 1976). Anthocyanins are pigments responsible for petal colors in sweet pea (Pecket, 1966). Our research group recently isolated and identified 2-CEIX as a compound that reduces anthocyanin coloration from sweet pea petals by a different isolation method from the present study (Shimizu-Yumoto et al., 2012).
Although 2-CEIX has been found in various organs, including petals, stem and leaves in sweet pea (Ikegami et al., 1984), it has been unclear whether 2-CEIX is a major nitrogenous compound in sweet pea. In the present study, thus, the contents of amino acids and other nitrogenous compounds as well as 2-CEIX contents in the petals, stems and leaves were determined. Asparagine and α-alanine were found to be major amino acids in the petals, leaves and stem (Table 1). The 2-CEIX content was the third highest nitrogenous compounds in the petals. In stem and leaves, 2-CEIX contents were much higher than other nitrogenous compounds. These findings clearly indicate that 2-CEIX is a major nitrogenous compound in sweet pea. The contents of 2-CEIX were much higher in these organs than in roots as reported by Kuo et al. (1982). The contents of 2-CEIX in the stem and leaves were much higher in the present study than in those reported by Ikegami et al. (1984). The differences may be attributed to the method of quantification. In the study of Ikegami et al. (1984), the 2-CEIX content was determined by an amino acid analyzer. However, 2-CEIX contents might not be determined precisely in the study of Ikegami et al. (1984) because 2-CEIX is not an amino acid. Alternatively, there may be cultivar variations with 2-CEIX contents in sweet pea.
For flower opening, large amounts of soluble carbohydrates are required as substrates for respiration and synthetic materials as well as osmotica (Yamada et al., 2009). The 2-CEIX content in the petals was high, particularly at stage 1 (Fig. 2). The 2-CEIX content in the petals was comparable to other soluble carbohydrates, suggesting that 2-CEIX may act as an osmoregulator in sweet pea petals. However, the 2-CEIX content decreased, but monosaccharide and sucrose contents increased markedly during flower opening (Fig. 2A). Thus, 2-CEIX appears to be less important than monosaccharides and sucrose for flower opening. In rose flowers, fructose and glucose mainly accumulate in the vacuole in rose petals, and contribute to petal cell expansion associated during flower opening (Yamada et al., 2009). Subcellular contents of 2-CEIX in sweet pea petals should be investigated to clarify its function for cell expansion.
We found that the total contents of amino acids and nitrogenous compounds were much higher in the petals than in the stem and leaves (Table 1), suggesting that nitrogenous compounds may be important in sweet pea petals. The total content of nitrogenous compounds in the petals is estimated to be 89 μmol·g−1FW, which was as high as monosaccharide content. Thus, nitrogenous compounds appear to contribute to maintaining osmotic pressure in sweet pea petals. In sandersonia petals, glutamine, aspargine, glutamic acid and aspartic acid are major amino acids (Eason et al., 2000). In the petals of Eustoma grandiflorum, glutamine and asparagine are major amino acids, and some other amino acids, such as aspartic acid, glutamic acid and serine, are minor compounds (Kawabata and Chujo, 2008). However, the total amino acid content in the petals is estimated to be much higher in sweet pea than in sandersonia or E. grandiflorum.
Nitrogen fixed in the roots is transported through xylem in legume plants (Pate et al., 1979; Peoples et al., 1986). Since 2-CEIX is the major nitrogenous compound in sweet pea roots, we had hypothesized that 2-CEIX is transported from roots to the other organ through xylem. However, 2-CEIX was not found in xylem saps, suggesting that 2-CEIX is not transported through xylem. In some legume plants, including soybean (Matsumoto et al., 1978; Shelp and Da Silva, 1990) and Vigna unguiculata (Pate et al., 1984), fixed nitrogen is transported as ureides through xylem. In other legume plants, including Pisum sativum (Urquhart and Joy, 1981) and Lupinus albus (Jeschke et al., 1986), glutamine and asparagine are major translocated amino acids. Since asparagine is a major amino acid in sweet pea root (Kuo et al., 1982), it might be transported in xylem.
In legume plants, including soybean (Pate et al., 1984), Pisum sativum (Urquhart and Joy, 1981) and Lupinus albus (Jeschke et al., 1986), glutamine and asparagine are major translocated amino acids through phloem. We investigated whether 2-CEIX is transported through phloem or not. Tracer experiments using 14C showed that 14C-2-CEIX was not found, but 14C-sucrose was only detected in phloem exudates (Fig. 5). This suggests that 2-CEIX is not a substance for translocation, but sucrose is a carbohydrate for translocation in sweet pea. It is well known that sucrose is a carbohydrate for translocation in many plants (Zimmermann and Ziegler, 1975).
In the present study, 2-CEIX was detected in all the organs examined at relatively high contents (Table 1), and the 2-CEIX content in the petals increased 6-fold during flower development (Fig. 2B). Furthermore, 2-CEIX contents in the petals and other floral parts of cut flowers increased regardless of sucrose treatment (Figs. 3 and 4). We also showed that 2-CEIX is not transported through xylem or phloem. These results suggest that 2-CEIX may be synthesized in various organs. Biosynthetic pathways of some isoxazoline compounds in Streptomyces have been studied. Ornithine is a candidate as a precursor in Streptomyces sviceus (Gould and Ju, 1992), whereas arginine and serine have been proposed to be precursors in S. lavendulae (Uda et al., 2013). However, basic biosynthetic pathways of 2-CEIX seems different even if amino acids or polyamines are prime precursors because of difference in their ling part structures.
Nitrogen in senesced leaves remobilizes to other tissues (Masclaux-Daubresse et al., 2010). Similarly, nitrogen remobilizes in the petals of petunia during senescence (Shibuya et al., 2013). Although the 2-CEIX content in sweet pea leaves during senescence has not yet been investigated, the 2-CEIX content in the petals and the other floral parts increases during senescence (Fig. 3). Thus, 2-CEIX seems not to be a reserve compound. 2-CEIX is an alkaloid, which belongs to diverse group of low molecular weight, nitrogen-containing compounds (Ziegler and Faccini, 2008). Plants are estimated to produce approximately 12000 different alkaloids (Ziegler and Facchini, 2008). Many alkaloids are toxic compounds that play roles as defensive chemicals against herbivores (Kutchan, 1995). Some isoxazoline compounds, including fluralaner, have been shown to be toxic to insects (Gassel et al., 2014). Although the toxicity of 2-CEIX to insects is unclear, 2-CEIX has been shown to be toxic to rats (Van Rompuy et al., 1974b). We propose that 2-CEIX accumulates in sweet pea plants as a defensive compound from herbivores, and its accumulation at high levels may contribute to maintenance of osmotic potential.
In conclusion, 2-CEIX was identified as the nitrogenous compound of low molecular weight in sweet pea. The contents of 2-CEIX in the petals, stem and leaves were relatively high, which was comparable to amino acid contents. 2-CEIX was not found in xylem or phloem saps, suggesting that this compound is synthesized in situ in various organs. In cut sweet pea, the 2-CEIX content in the petals increased with time, and this increase continued by sucrose treatment. These trends differed from those of monosaccharides, sucrose and cyclitols. Although its precise role is unclear, 2-CEIX is present constitutively as a major nitrogenous compound in various organs of sweet pea.
We thank the late Mr. Y. Yamaguchi and the late Dr. Y. Mukasa for their technical assistance in this work.