2016 Volume 85 Issue 2 Pages 154-160
2016 Volume 85 Issue 2 Pages 154-160
We investigated morphological changes in petal cells during flower development and opening in Eustoma grandiflorum. The morphology of petal epidermal cells was observed by scanning electron microscopy, and their number was determined. The numbers of adaxial and abaxial epidermal cells increased during flower development. Increase in these numbers terminated before flower opening earlier in abaxial than in adaxial epidermal cells. Measurements of cell number and area showed that the petal growing stage during flower development and opening can be divided into four phases: cell division and expansion, cell division, cell division and expansion, and cell expansion. Adaxial epidermal cells in the petal blade showed a conical-papillate shape whereas adaxial epidermal cells in the petal claw were longitudinally elongated in shape. Abaxial epidermal cells were longitudinally elongated in both petal blade and claw. The ultrastructure of petal cells at the bud stage and the open stage was observed by transmission electron microscopy. In the petal cells at the bud stage, nuclei and several plastids were observed, although the cells were mainly occupied with vacuoles. Relatively large spherical electron-dense bodies were observed only in the vacuoles of adaxial epidermal cells at the bud stage. The petal cells were largely occupied with enlarged vacuoles at the open stage. We conclude that petal growth in Eustoma is divided into four phases, based on the activities of cell division and expansion, and that petal growth in the final phase is mainly due to cell expansion with marked enlargement of vacuoles.
Eustoma grandiflorum is native to North America and was introduced to Japan approximately 80 years ago. Physiological studies of flowering have enabled its year-round production (Katsutani, 2006), and numerous cultivars with various floral colors, sizes, and shapes have been produced. Thus, Eustoma has become a major ornamental plant mainly used as cut flowers. Inflorescences of Eustoma have many flower buds, but flower opening in cut Eustoma kept in water is often suppressed (Ichimura and Korenaga, 1998; Shimizu and Ichimura, 2005). To extend the vase life of cut Eustoma, promotion of slow flower opening is desirable.
Petal growth associated with flower opening consists of both petal cell division and cell expansion. In carnation flowers, increase in DNA content terminated when petals emerged from the calyx, suggesting that cell division stops at this stage (Kenis et al., 1985). In Gaillardia grandiflora (Koning, 1984) and Tweedia caerulea (Norikoshi et al., 2013), the division of petal cells stopped before flower opening and petal growth associated with flower opening was due to cell expansion. However, cell division in rose did not stop in open flowers, and petal growth during flower opening was due mainly to cell expansion (Yamada et al., 2009b).
Morphological studies of petal growth during flower opening are limited in number. For petal cell expansion, accumulation of osmotica in the cells is required. Soluble carbohydrate concentrations in the petals increase during flower opening in many flowers, including carnation (Ichimura et al., 1998), rose (Yamada et al., 2009a), and T. caerulea (Norikoshi et al., 2013), suggesting that soluble carbohydrates act as osmotica. In rose petal cells, soluble carbohydrates accumulate in vacuoles, resulting in decreased osmotic potential, which is associated with cell expansion during flower opening (Yamada et al., 2009a). Expansion of petal cells during flower opening in rose is associated with enlargement of vacuoles and disappearance of plastids (Yamada et al., 2009a, b).
Morphological studies of petal cells during flower opening would contribute to the regulation of petal growth associated with flower opening. Kawabata et al. (2011) reported that cell expansion was involved in petal growth during flower opening in Eustoma. However, it remains unclear whether the division of petal cells stops during flower development. Moreover, the morphology of petal cells during flower opening has not previously been investigated in Eustoma. In the present study, we observed the morphology of adaxial and abaxial epidermal cells in petals using scanning electron microscopy (SEM). We investigated the stage when cell division terminated by counting epidermal cells. We also investigated the ultrastructural morphology of petal cells during flower development and opening in Eustoma by transmission electron microscopy (TEM).
Eustoma grandiflorum ‘Asuka-no-Nami’ flowers were grown in a greenhouse under natural day length conditions. As shown in Figure 1, petals at the following nine stages were sampled: stage 1, buds ca. 4 mm in length; stage 2, buds ca. 8 mm in length; stage 3, buds ca. 13 mm in length; stage 4, buds ca. 18 mm in length; stage 5, buds ca. 21 mm in length; stage 6, buds ca. 26 mm in length; stage 7, buds ca. 43 mm in length; stage 8, buds ca. 46 mm in length and pigmentation was started; and stage 9, on the day of anthesis. The petals from each flower were sampled and used immediately for experiments.

A. Photographs showing developmental stages of Eustoma grandiflorum flower. B. Petal area during flower development and opening. Values are the means of three independent experiments ± SE.
The total area of petals was measured with a leaf area counter (AAM-9; Hayashi Denko, Tokyo, Japan) within 2 h after sampling.
Measurements of fresh weight and dry weightAt each stage, flowers were cut below the receptacle and fresh weight (FW) of whole flowers and petals was determined. At each stage, 15 flowers were used. Petals collected from five flowers were dried in an oven at 120°C for more than 3 days and their dry weight (DW) was determined.
Scanning electron microscopyPetal epidermal cells were observed using a scanning electron microscope (VE-7800; Keyence, Tokyo, Japan), according to the instruction manual. The cell numbers of five angles randomly selected from one petal were counted with the area of one angle being 22059 μm2. Total cell number per petal was calculated by multiplying the counted cell number by the total petal area. The average cell area was determined by one angle area divided by the cell number, as described by Nishijima et al. (2006).
Transmission electron microscopyThe petal tissues at stages 6 and 9 were fixed in a mixture of 3% glutaraldehyde and 1% paraformaldehyde in 0.1 M phosphate buffer (pH 7.2) at room temperature for 5 h. After washing with rinsing buffer (0.2 M phosphate buffer, pH 7.2), the samples were post-fixed in 1% osmium tetroxide in 0.1 M phosphate buffer at 4°C for 2 h, dehydrated in a graded alcohol series, and embedded in epoxy resin. Ultrathin sections were prepared with diamond knives on a SUPER NOVA microtome (LKB, Stockholm, Sweden). The sections were placed on grids and stained with 2% aqueous uranyl acetate, followed by a lead electron staining solution (Hanaichi et al., 1986). The sections were observed using a transmission electron microscope (JEM 1200EX; JEOL, Tokyo, Japan).
Figure 1 shows the area of petals during flower development and opening. Petal area increased exponentially during flower development.
Figure 2 shows the FW of whole flowers and petals and the DW of petals during flower development. Although the FW of whole flowers and petals exponentially increased during flower development, the ratio of petal weight to whole flower weight increased. The DW of petals also increased during flower development. The FW/DW ratio in petals increased until stage 8 but remained constant thereafter.
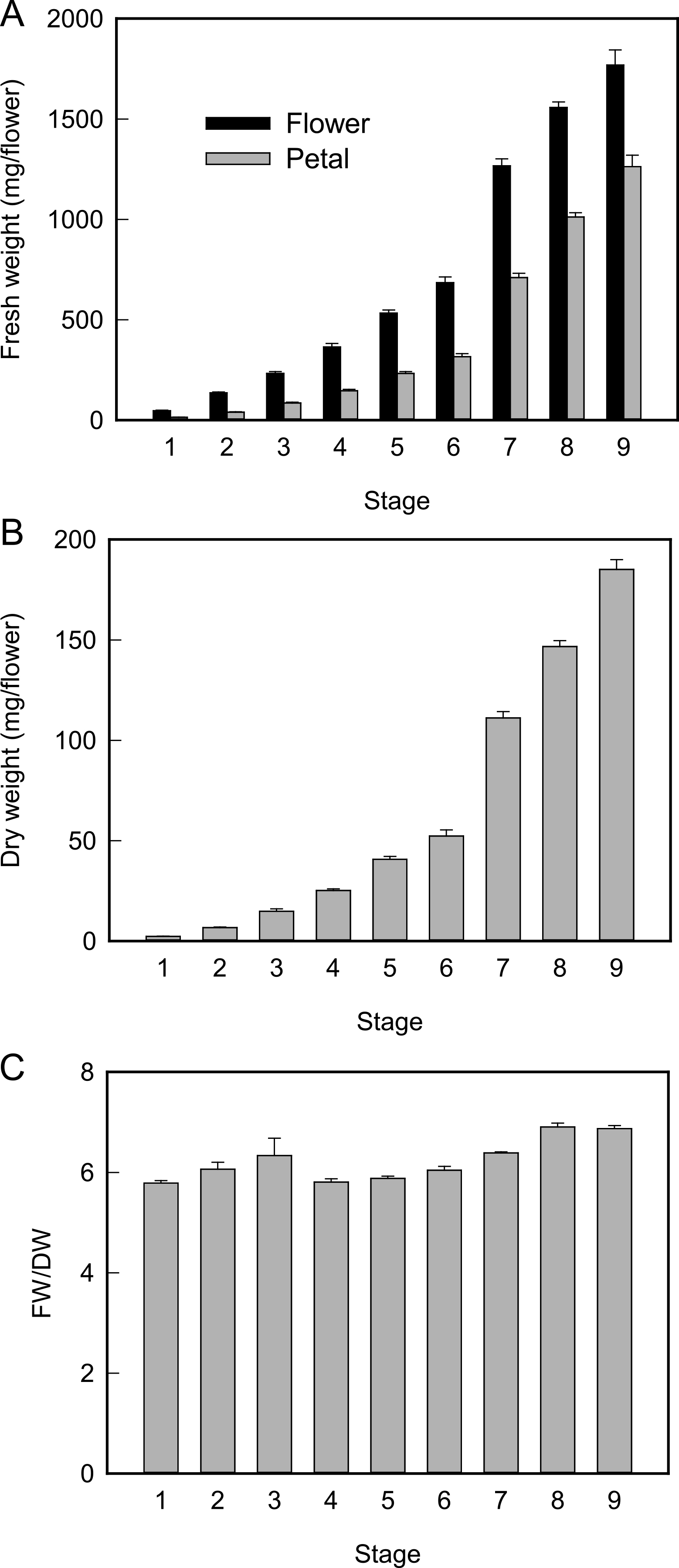
Fresh weight of whole flower and petal (A), dry weight of petal (B), and fresh weight/dry weight of petal (C). Values are the means of 10 (A, B) or three (C) independent experiments ± SE.
The abaxial epidermal cell area was larger than the adaxial one at all stages, particularly stages 1 and 2 (Fig. 3A). The areas of adaxial and abaxial epidermal cells increased considerably until stage 4. The areas of both cells were almost constant between stages 4 and 6, and markedly increased thereafter.

Adaxial and abaxial cell area (A) and cell number (B) of petals during flower opening. Values are the means of three independent experiments ± SE.
The adaxial epidermal cell number was much greater than the abaxial one. The number of adaxial epidermal cells increased and reached a plateau at stage 8. Similarly, the number of abaxial epidermal cells increased until stage 7 (Fig. 3B). This observation suggests that cell division stops earlier in abaxial than in adaxial epidermal cells.
Morphology of epidermal cells during flower openingMarked differences in the morphology of adaxial epidermal cells were observed between the petal blade and claw. Adaxial epidermal cells in the petal blade showed a conical shape at stage 1. Adaxial epidermal cells adopted a typical conical-papillate shape during flower opening (Fig. 4). Abaxial epidermal cells in the petal claw were elongated.

Scanning electron micrographs of adaxial and abaxial epidermal cells of petals. (A) Adaxial epidermal cells in petal blade at stage 3 (A), stage 6 (B), and stage 9 (C). Adaxial epidermal cells in petal claw at stage 3 (D), stage 6 (E), and stage 9 (F). Abaxial epidermal cells at stage 3 (G), stage 6 (H), and stage 9 (I). Scale bars represent 50 μm.
The morphology of abaxial epidermal cells markedly differed from that of adaxial epidermal cells. Adaxial epidermal cells in the claw at stage 1 were elongated, irrespective of petal parts. Although the sizes of abaxial epidermal cells increased during flower opening, their cell shape changed little during flower opening.
Ultrastructure of adaxial petal cells during flower openingTo investigate the morphology of adaxial petal cells in detail, the ultrastructure of petal cells at stages 6 and 9 was observed using TEM. Although petal cells were mainly occupied by vacuoles, some organelles, including nuclei and plastids with starch granules, were observed at stage 6 (Fig. 5). Moreover, spherical electron-dense bodies were observed in vacuoles of adaxial epidermal cells at stage 6. At stage 9, cells were mostly occupied by vacuoles, and spherical electron-dense bodies were not observed.

Transmission electron micrographs of adaxial petal cells at stage 6 (A) and stage 9 (B). Scale bars represent 5 μm.
In Eustoma flowers, petal FW and DW exponentially increased during flower opening and FW/DW ratio did not increase between stages 8 and 9 when flowers opened (Fig. 2). In other flowers including daylily (Bieleski, 1993), rose (Evans and Reid, 1988), and T. caerulea (Norikoshi et al., 2013), the FW/DW ratio increases during flower opening, suggesting that petal growth involves water influx in these plants. No decrease in the FW/DW ratio in petals during flower opening has been reported for other flowers. We observed that an unknown sticky substance was secreted on the basal parts of petals at stage 9 in Eustoma. The marked increase in DW may be due to this substance.
Although petal growth associated with flower opening involves the expansion of petal cells (Kawabata et al., 2011), the manner of cell division has not previously been investigated in Eustoma. In the present study, we investigated the numbers of petal epidermal cells during flower development and opening using SEM. The number of abaxial epidermal cells did not increase from stage 7, whereas that of adaxial epidermal cells almost reached a plateau at stage 8 (Fig. 3), suggesting that cell division stops earlier in abaxial than in adaxial epidermal cells. Cell division of adaxial and abaxial epidermal cells terminates at the same stage in T. caerulea (Norikoshi et al., 2013). In contrast, the division of both adaxial and abaxial epidermal cells in rose continues even when flowers have opened completely (Yamada et al., 2009b). Thus, patterns of cell division and expansion during flower opening vary depending on the plant species.
Adaxial and abaxial epidermal cell area and cell number increased considerably between stages 1 and 4 and between stages 6 and 8 (adaxial cell) or stages 6 and 7 (abaxial cell), indicating that petal growth is due to cell division and expansion during these stages. In contrast, epidermal cell area did not increase, but cell number increased between stages 4 and 6, suggesting that petal growth during these stages is mainly due to cell division. Adaxial and abaxial epidermal cell area increased between stages 8 and 9 and stages 7 and 9, respectively, but cell number did not increase, suggesting that petal growth is due to cell expansion. Thus, the petal growing stage during flower development and opening can be divided into four phases in Eustoma: cell division and expansion, cell division, cell division and expansion, and cell expansion. In G. grandiflora (Koning, 1984) and T. caerulea (Norikoshi et al., 2013), only two phases have been observed: cell division and expansion, and cell expansion. In rose petals, similar findings have been reported, although cell division continues in the final stage of flower opening (Yamada et al., 2009b). It is unclear whether the presence of the four phases is specific to Eustoma. In the present study, petal cell number and area were investigated in very immature buds at several points, in contrast to previous studies. If petal growth is investigated in more immature stages at more points in other flowers, then similar results may be observed.
We measured cell number and size using whole petals. Thus, it is unclear whether cell division and expansion progress simultaneously at various petal parts. Reale et al. (2002) investigated cell division of petals by in situ hybridization using two markers, cyclin and histone 4 genes, during flower development in petunia. They showed that cell divisions are uniformly distributed throughout the petal initially and decline gradually, starting from the basal part (Reale et al., 2002). By using their method, modes of cell division and expansion can be understood in detail in Eustoma petals.
Adaxial epidermal cells in the petal blade showed conical shapes at stages 1 and 2 and gradually changed to a conical-papillate shape at later stages (Fig. 4). In the petals of several other flowers, such as morning glory (Yoshida et al., 2003), rose (Yamada et al., 2009b), snapdragon (Jackson et al., 1992), and wallflower (Weston and Pyke, 1999), adaxial epidermal cells also show conical-papillate shapes at the open stage. Changes in cell shape during flower opening have also been reported in rose (Yamada et al., 2009b) and wallflower (Weston and Pyke, 1999).
Marked differences in the shape of adaxial epidermal cells were observed between petal blade and claw (Fig. 4). As observed in abaxial epidermal cells, adaxial epidermal cells in the petal claw showed elongated shapes. Similar morphology has been observed in torenia (Niki et al., 2012). In Arabidopsis petals, adaxial and abaxial epidermal cells show similar morphology (Pyke and Page, 1998). Kawabata et al. (2011) reported differences in cell expansion between petal blade and claw. These differences may be involved in different cell shapes. However, the morphology of cells at the junction of blade and claw is unclear. Petunia petals are composed of limb and tube, which are equivalent to blade and claw, respectively, in Eustoma petals. Reale et al. (2002) investigated cell width and length in different portions of petunia petals during flower development. Their results suggest that the shape of tube cells on the apical side relatively well resembles the shape of limb cells, compared with the shape of tube cells on the basal side. On the basis of the finding of petunia petal cell morphology, we suppose that the junction of cells has morphology that is intermediate between those of blade and claw.
Abaxial epidermal cells were elongated in shape at all stages (Fig. 4), suggesting that cell shape is largely determined at a very early stage of floral development. This type of cell has not been observed in petals of other flowers; abaxial epidermal cells in carnation (Fukai et al., 2007), T. caerulea (Norikoshi et al., 2013), and wallflower (Weston and Pyke, 1999) were shown to be lenticular in shape. Further studies may establish whether this cell shape is specific to Eustoma.
In Eustoma petals, several plastids containing starch granules were observed in epidermal and parenchyma cells in petals at stage 6, but plastids were infrequently observed in petals at stage 9 (Fig. 5). This finding suggests that stored starch is degraded to soluble carbohydrates because large amounts of soluble carbohydrates are required for flower opening in Eustoma (Shimizu and Ichimura, 2005). The disappearance of plastids has also been observed in rose petals (Yamada et al., 2009b). Similarly, a decrease in starch content in petals has been reported in rose (Evans and Reid, 1988; Ho and Nichols, 1977) and gladiolus (Yamane et al., 1991). In cut rose petals, stored starch is degraded to hexose during flower opening, contributing to decreased osmotic potential and facilitating the water influx required for cell expansion (Evans and Reid, 1988).
Relatively large, spherical, electron-dense bodies were observed only in vacuoles of petal adaxial epidermal cells at stage 6 (Fig. 5). Anthocyanic vacuolar inclusions have been observed in vacuoles of adaxial epidermal cells in Eustoma, but they were not spherical (Markham et al., 2000). Moreover, the spherical structures were observed in non-pigmented petals (stage 6), but typical spherical structures were not observed in pigmented petals (stage 9; Fig. 5). These findings suggest that spherical bodies observed in the vacuole are not anthocyanic vacuolar inclusions. Protein bodies, which are spherical, have been observed in vacuoles in some petals including petunia, tomato, tobacco (Shumway et al., 1972), and Rhododendron (Schneider, 1972). However, intravacuolar protein bodies have not been observed in petals in carnation (Smith et al., 1992) and rose (Yamada et al., 2009b). Similarly, spherical bodies were not observed in Delphinium, gentian, and snapdragon sepals or petals in preliminary experiments (unpublished data). In Rhododendron petals, protein bodies were mainly observed in epidermal cells at the bud stage (Schneider, 1972). This distribution and stage are common to spherical electron dense structures found in Eustoma petals. Thus, we propose that these structures are protein bodies and reserve amino acids required for protein synthesis for cell expansion.
Cell expansion is regulated by not only the accumulation of osmotica and water influx but also the strength of cell walls (Boyer et al., 1985; Cosgrove, 2001). Numerous proteins and enzymes are required for cell expansion. Genes encoding expansin, which is involved in cell wall loosening, are expressed during flower opening of carnation (Harada et al., 2011), Mirabilis jalapa (Gookin et al., 2003), and Petunia hybrida (Zenoni et al., 2004), and xyloglucan endotransglycosylase/hydrolase (XTH) activity is associated with the opening of sandersonia flowers (O’Donoghue et al., 2002). Recently, Ochiai et al. (2013a) reported that the expression of expansin and XTH genes and their proteins increased during flower opening in Eustoma. Treatment with methyl jasmonate promoted flower opening, which was associated with increases in XTH and expansin (Ochiai et al., 2013b). These findings suggest that XTH and expansin are important for flower opening in Eustoma. To investigate the relationship between morphological changes in petal cells and gene expression, the role of genes in cell expansion should be understood.
In conclusion, petal growth in Eustoma during flower bud development and opening can be divided into four phases: cell division and expansion, cell division, cell division and expansion, and cell expansion. Marked differences in morphology were observed between adaxial and abaxial epidermal cells. Adaxial epidermal cells showed expansion with enlargement of the vacuole during flower opening.
We thank Prof. K. Suzuki (Shizuoka University) for his advice. We also thank Dr. N. Fukuta and Ms. K. Kataoka (NIFS) for cultivating the Eustoma plants.