2016 Volume 85 Issue 4 Pages 360-365
2016 Volume 85 Issue 4 Pages 360-365
To assess the growth and flowering of the Doritaenopsis orchid in alternative substrates, Doritaenopsis Queen Beer ‘Mantefon’ was grown for 15 months in four different substrates; a commercial 100% Chilean sphagnum moss (S), peat moss (P), medium-grade Douglas fir bark mixed with P with a 3:7 ratio (v/v) (BP), and S and P mixes (SP) with a 4:6 ratio (v/v). Physical (porosity and water holding capacity) and chemical (pH and EC) properties of the four substrates were investigated. SP substrate had significantly higher substrate volumetric water content (VWC) than the other three substrates only for the first 3 days after fertigation, and only the BP substrate maintained lower air space than the other substrates. Although there was no relevant growth responses to VWC and air space changes, a better growth in shoots and fastened flowering of Doritaenopsis ‘Mantefon’ occurred in plants grown in the P substrate, which could be attributed to providing better contact of terrestrial roots to the substrate enabling enough water and nutrient supply, along with a proper pH range of 6.15. At 15 months after transplanting, plants grown in the P and BP substrates had larger leaves and a greater shoot dry weight than the plants grown in the S and SP substrates. Plants grown in the P substrate produced 2.75 flower spikes, whereas the plants grown in the S, BP, and SP substrates produced 2.00 to 2.33 flower spikes. Plants grown in the P, BP, S, and SP substrates produced a third flower spike, being 67%, 33%, 17%, and 8%, respectively. There was no significant difference in the total number of flowers, while the total numbers of buds were 32.3, 23.4, 23.0, and 19.7 in plants grown in the P, S, BP, and SP substrates, respectively. Time to visible flower spike was shortened in plants grown in the P substrate compared to the plants grown in other substrates. With these results, using alternative substrates including peat moss for Doritaenopsis cultivation, growers may be able to promote leaf growth and flower spike induction with lower expenses on substrate costs, resulting in a high quality production of Doritaenopsis ‘Mantefon’ with more profit.
Phalaenopsis orchids have been cultured for well over 100 years throughout the world, and worldwide orchid production in 2014 reached more than 300 million pots, with the majority being Phalaenopsis. In Taiwan, the export value of the Phalaenopsis orchid in 2011 increased by more than US$ 10 million over the same period in 2010 (Council of Agriculture, 2015; http://eng.coa.gov.tw/content_view.php?hot_new=8790&catid=24200; 16 September, 2015). In the United States, orchids mainly represented by Phalaenopsis, ranked first in wholesale value in the potted flowering plant market in 2011 (U.S. Department of Agriculture, 2012; http://usda.mannlib.cornell.edu/usda/nass/FlorCrop//2010s/2012/FlorCrop-05-31-2012.pdf; 4 February, 2016), and Phalaenopsis consisted of 68.7% of the orchid auction in the Korean flower market in 2013 (Korea Agro-Fisheries and Food Trade Corporation, 2014; http://yfmc.at.or.kr/article/fmko342000/view.action?articleId=20163; 7 July, 2015). Phalaenopsis is an epiphytic, monopodial orchid native to southeastern Asia. As a result of extensive hybridization, these orchids have become available in various flower sizes and diverse colors (Vasquez and Frier, 1991). Recently marketed Doritaenopsis is an intergeneric hybrid of Doritis and Phalaenopsis, and market demand for Doritaenopsis is increasing because they have a longer flowering period with a variety of flower colors and shapes.
Phalaenopsis species including Doritaenopsis are generally grown in several different potting substrates, including activated charcoal, chopped coconut husks and fiber, cork, Douglas fir bark, peat moss, perlite, redwood bark, rock wool, sphagnum moss, and tree fern fiber (Blanchard and Runkle, 2008; Wang, 2005). Among these substrates, the primary orchid substrate component used in the United States and Europe is Douglas fir bark (Blanchard and Runkle, 2008; Wang, 2000), while sphagnum moss is the most common substrate for orchid production in Eastern Asia including Korea, Taiwan, and Japan (Hwang and Jeong, 2007).
Sphagnum moss is a group of mosses belonging to the Sphagnum genus. Dead cells of sphagnum moss are large in volume with thin but firm cell walls, which are excellent for transmitting water and holding shape (Puustjarvi, 1977). These characteristics make it an ideal substrate to retain water and air for epiphytic orchids including Phalaenopsis (Yao et al., 2008). However, in Korea, sphagnum moss is generally imported from New Zealand, Chile, or Japan, resulting in higher costs on a commercial scale. Higher labor costs were also required since plants needed to be tightly potted in sphagnum moss for better water and nutrition uptake (Hwang and Jeong, 2007). These expensive production costs of Phalaenopsis with sphagnum moss have inspired a search for alternative substrates to sphagnum moss for more profitable production. Developing inexpensive but effective potting substrate alternatives can potentially reduce the fertilization rate, irrigation rate, and ultimately, growth costs for producing orchids. Furthermore, inconsistent sphagnum moss supplies is another reason to seek sphagnum moss alternatives for use in orchid production in Eastern Asia.
Wang (1995) reported that Phalaenopsis plants grown in a mixture of commercial substrate mix (Metro Mix 700; Hummert Intl., Earth City, MO, USA), perlite, and horticultural-grade charcoal produced twice the number of new leaves and had a 2.5-fold larger leaf area than those grown in large bark, suggesting the smaller particles of peat moss in the substrate provided good contact with roots, promoting fast recovery from transplanting and rapid growth of Phalaenopsis plants compared to using coarse bark only as a substrate. Although Wang and Konow (2002) also suggested peat moss mixed with bark for growing Phalaenopsis, sphagnum moss was not included in their substrate comparison study. Hwang and Jeong (2007) reported that two Phalaenopsis cultivars ‘Stripe’ and ‘White Red Lip’ grown in coir mixes partially mixed with sphagnum moss had more leaves and greater fresh/dry weight than those grown in sphagnum moss, but flowering responses to different potting substrates were not provided in their results.
Physical characteristics such as air porosity and moisture content are critical factors for orchid plants grown in containers, which are affected by substrate composition. Proper pore distribution in the substrate is critical, since a substrate with macropores cannot retain an adequate amount of nutrients and water for optimum plant growth and provide good aeration, whereas substrate micropores can easily hold water and nutrients, although excessive water can result in root rot and fungal infection (Raviv and Lieth, 2008). Understanding the physical and chemical characteristics of potting substrates, which greatly affect plant growth, is essential when looking for sphagnum moss alternatives in orchid mass production in East Asia. The objectives of this study were to investigate the physical and chemical characteristics of four potting substrates for Doritaenopsis production, and to examine the effects of different substrates on the growth and flowering of Doritaenopsis.
Clones of tissue culture propagated Doritaenopsis Queen Beer ‘Mantefon’ (East Sky Biotech, Dongducheon, Gyeonggi, Korea) at 9 months old with 3–4 fully developed leaves were transplanted to 12 cm plastic pots filled with four different substrates. The four potting substrates were Chilean sphagnum moss (S), peat moss (P; Klasmann-Deilmann GmbH, Geeste, Germany), medium-grade Douglas fir bark (Klasmann-Dellmann GmbH) mixed with P with 3:7 ratio (v/v) (BP), and S mixed with P at a 4:6 ratio (v/v) (SP). The pH value of peat moss was adjusted by using fast reacting lime and a high magnesium content. All plants were placed in a growth chamber (HB-301MP; Hanbaek Scientific Co., Ansan, Korea) with a temperature of 29°C, 12/12 h (light/dark) photoperiod with 120 ± 10 μmol·m−2·s−1 at the canopy level with high pressure metal halide lamps (MH250W; Hanyoung Electric Co., Hwasung, Korea) and three-wave cool white fluorescent lamps (EFTR20EX-D; Hangzhou Li-Tech Electric Co., Ltd., Hangzhou, China). Relative humidity in the growth chamber was maintained at 60% during the experimental period. The plants were fertigated once every 10 days by hand with a solution of water soluble fertilizer (EC 0.8 ± 0.1 dS·cm−1 and pH 6.0–6.5; Newgold 20N-8.7P-16.7K; Neufarm Corp., Ltd., Munster, Germany).
Physical characteristics of the four substratesTo determine the physical properties of the four substrates, their four replicates were filled in specific aluminum cylinders, and the procedures of Fonteno and Bilderback (1993) were followed. Four samples were used to determine total porosity, container capacity, air space, and bulk density. The function was defined as: container capacity (CC) = ((wet weight—dry weight)/volume of sample) × 100, air space (AS) = (volume of water drained/volume of sample) × 100, and total porosity (TP) = CC + AS. Bulk density was calculated by dividing dry weight (24 h at 105°C) by volume (Klute, 1986). pH and EC of the four substrates were measured following saturated media extraction methods with a pH meter (Accumet model 20; Fisher Scientific, Pittsburgh, PA, USA) and an electrical conductivity meter (Orion 3 Star; Thermo Fisher Scientific Inc., Beverly, MA, USA). Substrate volumetric water content (VWC, v/v) changes over 8 days after fertigation application were measured with dielectric soil moisture sensors (WaterScout SM 100 Sensor; Spectrum Tech. Inc., Aurora, IL, USA) connected to a datalogger (WatchDog 1000 Series Micro Stations; Spectrum Tech. Inc.) after substrate-specific VWC calibrations.
Growth and flowering parametersThe time to visible flower spike from the start of the treatments was measured when the flower spike was 0.5 cm in length. The time at which the labellum in the first floret was fully open was regarded as the flowering time. At harvest, 15 months after transplanting, total number of leaves, leaf length, and width of fully expanded leaves were measured for each plant. Chlorophyll content was measured using a chlorophyll meter (SPAD 502; Minolta Camera Co., Ltd., Osaka, Japan) with the uppermost fully expanded leaves. The number and length of flower spikes and lateral flower spikes, total number of flower buds and flowers, and flower diameter were measured. Fresh weights of vegetative shoots and roots were measured at harvest and their dry weights were determined after drying samples in an oven at 80°C for 3 days.
Statistical analysisThe experimental design was a randomized complete block with three replications with 4 plants in 4 pots. Analyses of variance were conducted using the SAS system (version 9.3; SAS Institute Inc., Cary, NC, USA) and SigmaPlot (version 10; Systat Software Inc., San Jose, CA, USA). Differences among the treatment means were assessed by Tukey’s studentized range test at P < 0.05.
The P and SP substrates had the highest container capacity at over 80%, followed by the S substrate, and the lowest was 52.8% in the BP substrate (Table 1). The S substrate had the highest air space among the four substrates. Total porosity was 93.5%, 86.8%, 69.3%, and 92.2% in the S, P, BP, and SP substrates, respectively, and only the BP substrate had significantly lower total porosity than the S and SP substrates. Bulk density was different across the four substrates, and was highest in the BP substrate. The pH of the P substrate was the highest at 6.15 and the S substrate had the lowest pH at 4.81. There were no significant differences in EC among the four substrates ranging from 0.22 to 0.27 dS·m−1.
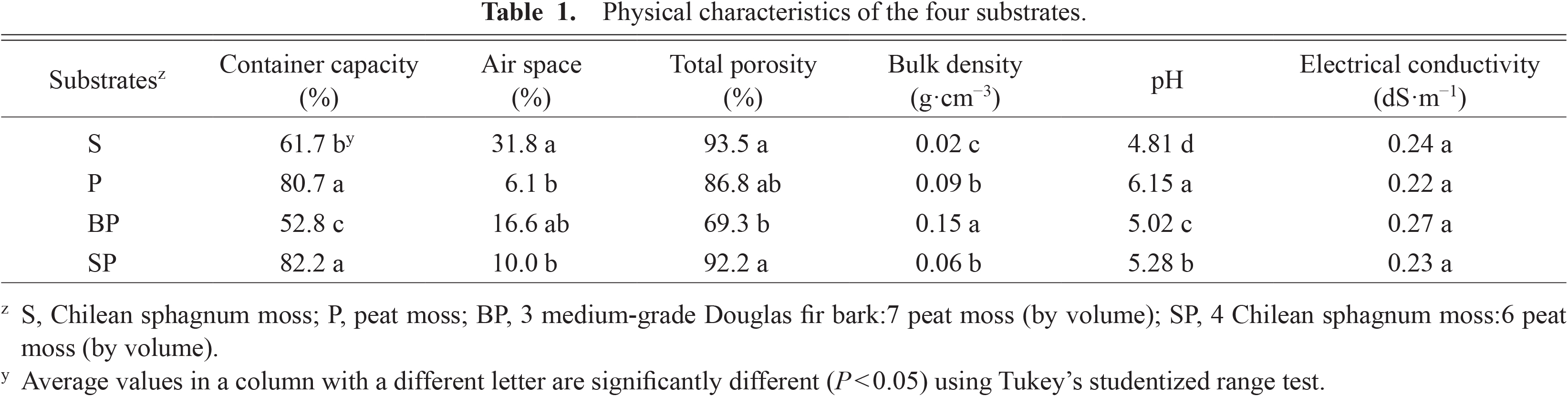
Physical characteristics of the four substrates.
After fertigation, VWC gradually decreased with time in all substrates (Fig. 1). VWC in the SP substrate was significantly higher than that in the P and BP substrates for the first 3 days after fertigation, but there was no significant difference in VWC among the four substrates from the 4th day after fertigation. As VWC decreased with time, the air space of the four substrates increased. Air space was significantly lower in the BP substrate than the other substrates for 8 days after irrigation, but there were no significant differences in air space among the S, P, and SP substrates.

The changes in volumetric water content (A) and air space (B) of four different soilless substrates after irrigation. Vertical error bars indicate standard errors (n = 3). S, Chilean sphagnum moss; P, peat moss; BP, 3 medium-grade Douglas fir bark:7 peat moss (by volume); SP, 4 Chilean sphagnum moss:6 peat moss (by volume).
Plants grown in the P and BP substrates had wider leaves than those grown in the S and SP substrates (Table 2). The highest leaf chlorophyll was obtained in plants grown in the P substrate, and the lowest in plants grown in the BP substrate. The dry weights of shoots in plants grown in the P and BP substrates were higher than those in the S and SP substrates, whereas the fresh and dry weights of roots showed no differences among the plants grown in different substrates. No significant differences were observed in the total number of leaves of Doritaenopsis ‘Mantefon’ at harvest across the four different substrates (Table 2).

Vegetative growth of Doritaenopsis ‘Mantefon’ at 15 months after transplanting in four different substrates.
Plants in the P substrate produced more flower spikes than those in the S and SP substrates (Table 3). Plants grown in the P substrate produced 2.75 flower spikes per plant, whereas those planted in the S, BP, and SP substrates produced 2.0 to 2.3 flower spikes (Table 3). Every single plant in four different substrates had the first flower spikes with no significant difference in spike length among the treatments, while the number of lateral branches was higher in plants grown in the P substrate than those grown in the S substrate. All plants in the P and BP substrates produced second flower spikes, whereas 92% of plants grown in the S or SP substrates produced second flower spikes. The length of the second flower spike and number of lateral branches showed no significant difference among the plants grown in different potting substrates. Sixty seven percent of the plants in the P substrate produced third flower spikes, whereas fewer than 33% of the plants in other substrates produced them. Only 8% of plants in the SP substrate produced a third flower spike. The length of the third flower spike in plants grown in the P substrate was 5 times longer than those in plants grown in the S substrate, and the number of lateral branches in the third flower spike was also higher in plants grown in the P substrate than those grown in the S and SP substrates (Table 3). Although plants grown in the P substrate produced substantially more flower buds than those in the other three substrates (Table 4), there was no significant difference in the total number of flowers among the plants in different substrates. Time to visible flower spike was the shortest in plants grown in the P substrate among the plants in the four different substrates. Flower diameter and time to flower were not affected by different substrates.

Flower spike growth of Doritaenopsis ‘Mantefon’ at 15 months after transplanting in four different substrates.

Flowering parameters of Doritaenopsis ‘Mantefon’ at 15 months after transplanting in four different substrates.
Most Phalaenopsis are epiphytic plants, natively growing on tree trunks and limbs with their roots exposed to air and absorbing moisture from the humid air. Due to these epiphytic characteristics, when growing Phalaenopsis in containers filled with soilless substrates, growers must consider good aeration and humid substrate moisture conditions, as well as the cost and consistency (Wang et al., 2005). The moisture tension of tightly packed sphagnum moss can remain at or higher than −20 kPa until 90% of the water is lost, thereby providing a higher amount of available water to roots (Wang, 2010). Since plants grown in a substrate with improved water retention do not need to be irrigated so frequently, less water and fertilizer is used (Wang, 1995). From these reasons, Wang et al. (2007) suggested that pure sphagnum moss may be the single best material for growing young Phalaenopsis in warm conditions, including tropical and subtropical areas. According to reports from Jin and Ichihashi (2002), Doritaenopsis potted with New Zealand or Chinese sphagnum moss grew better than those grown with New Zealand bark, coconut husk chips, or rock wool, and the growth differences among the five different potting substrates could be caused by their different water holding capacities and major ion release characteristics. However, in the present study, VWC was significantly higher in the SP substrate than in other substrates for 3 days after irrigation, but high quality Doritaenopsis ‘Mantefon’ with flower spikes was achieved by growing in the P substrate (Fig. 1; Tables 2–4). Furthermore, plants grown in the P substrate had a better vegetative growth, including wider leaves and greater shoot dry weight than plants grown in the S and SP substrates. Although it is not easy to conclude since we could not compare the VWC and air space changes over 15 months due to sensor availability, VWC and air space of the four substrates did not seem directly affect the growth, or flowering of Doritaenopsis ‘Mantefon’.
Acidification may be caused by root response to a lack of certain minerals in the rhizosphere, but this low pH does not seem to negatively affect plant growth or cause apparent toxicity from the accumulation of certain micronutrients in Doritaenopsis plants. Sphagnum moss is a natural material, which has numerous negatively charged sites that attract H+ (Yen et al., 2011). The decline in pH of sphagnum moss over time was commonly observed in the cultivation of Phalaenopsis (Hwang and Jeong, 2007; Yen et al., 2011). Although the low pH (< 4.4) in substrates did not appear to adversely affect plant growth (Wang and Gregg, 1994), water with a pH between 5.5 and 6.5 is recommended for irrigating Phalaenopsis for quality production (Gordon, 1990). In the present study, the P substrate had the highest pH at 6.15, which could provide better root conditions for growing Doritaenopsis ‘Mantefon’ than the S substrate with pH at 4.81. However, further experiments growing Doritaenopsis in substrates with different pH levels is expected to identify the appropriate substrate pH level for the species.
The term aerial root may be applied to any lateral root which develops and remains in free air. If aerial roots penetrate a substratum or attach themselves to a solid object, they serve as climbing or adhesive roots, fastening the flexible growing axis to some underlying surface; they may then perform like terrestrial roots, acting as organs of absorption (Dycus and Knudson, 1957). The fewer aerial roots and greater leaf span of plants grown in chunky peat suggested that root entry was relatively unrestricted and contact with roots was sufficient to provide adequate water and nutrients for leaf expansion (Blanchard and Runkle, 2008). The P substrate in the current research may have had good contact with roots and provided available water and nutrients better to the roots of Doritaenopsis. We also observed that plants grown in the P substrate produced fewer aerial roots than those grown in the S substrate (data not shown), which supports the notion that the P substrate had suitable physical characteristics for growing terrestrial roots of Doritaenopsis, resulting in larger leaves and more flower spikes than those grown in the other substrates.
In the present study, Doritaenopsis ‘Mantefon’ grown in the P substrate had the highest chlorophyll content and greater leaf size and biomass than plants grown in the S and SP substrates, and enhanced flower spike induction with more buds. Previous research indicated that bark and peat mix (7:3, v/v) produced more and larger Phalaenopsis leaves than bark medium alone (Wang and Konow, 2002), and our study also showed that Doritaenopsis ‘Mantefon’ grown in the BP substrate had larger leaf and greater biomass than the plants grown in the S or SP substrates. Another previous study indicated that Phalaenopsis with more leaves and larger leaf area produced a higher number of flower spikes and flowers than those with fewer leaves (Wang and Gregg, 1994), suggesting a good correlation between vegetative growth and flowering. Wang (2010) also reported that the capability of Phalaenopsis to respond to spiking induction temperatures (maturity) was directly related to plants’ total leaf area, with variety difference in the leaf area required to spike. Likewise, Doritaenopsis ‘Mantefon’ with larger leaves in the P substrate produced more flower buds and flower spikes than those in the other three substrates (Tables 3 and 4), indicating a better quality Doritaenopsis ‘Mantefon’ could be produced in the P substrate.
For most plant producers in Asia, the primary decision factor on which potting substrates to use most likely depends on economic factors. Potted flowering Doritaenopsis ‘Mantefon’ with more flower spikes are usually sold at a price 2 to 3 times higher in the Korean flower market than the price of plants with only one flower spike (An et al., 2013). Thus, Doritaenopsis ‘Mantefon’ with three flower spikes and with more buds is desirable for growers to improve their profits. Phalaenopsis orchids are sold by size (Wang and Konow, 2002), and orchid producers would benefit from a suitable substrate that can promote rapid leaf size increase. Also, it is critical that the time needed to produce flower spikes be shortened to reduce the cost and maximize profits for commercial growers. As well as high quality of Doritaenopsis ‘Mantefon’, peat moss is inexpensive compared to sphagnum moss—the current Korean wholesale price for peat moss is about half that of sphagnum moss. Our results indicated that Doritaenopsis ‘Mantefon’ grown in peat moss improved the production of leaves and flower spikes with more buds, and further shortened the flower spike induction than plants grown in sphagnum moss.
In conclusion, growing Doritaenopsis ‘Mantefon’ in peat moss may positively influence the plant quality such as leaf size and number of flower spikes, and also speeds up flower spike induction, reducing the production cost compared to growing the species in 100% sphagnum moss by reducing substrate and labor costs. However, re-potting is typical in long-term orchid growing in Asia, so further study with long-term time-series measurements of substrate and plant growth would be valuable in the future to observe changes over a long production time.