2017 Volume 86 Issue 1 Pages 45-51
2017 Volume 86 Issue 1 Pages 45-51
Contamination of vegetables with cadmium (Cd) is a worldwide problem. Three pot experiments were carried out to reduce the Cd content in fresh fruit of 52 Japanese pepper (Capsicum annuum L.) cultivars. The first experiment was carried out to select low Cd pepper cultivars after growing on a 2.1 Cd mg·kg−1 contaminated soil. The second experiment was carried out to select low Cd-accumulating pepper rootstocks while the third experiment was carried out to evaluate grafting as a tool to reduce Cd in fresh peppers. The ability of pepper cultivars to accumulate Cd in fresh fruit was significantly different. The Cd content ranged from 0.018 to 0.088 mg·kg−1 of fresh weight (FW). Among the pepper rootstocks cultivated in Japan, the cultivar ‘Daisuke’ was selected as a low-Cd accumulating rootstock. The grafting of cultivar ‘Ace’ onto ‘Daisuke’ rootstock reduced the Cd content by 40% in fresh peppers. However, other trace elements such as Fe, Mn, and Zn, which are important for human nutrition, were also reduced by 20%, 29%, and 42%, respectively.
Heavy metal contamination in agricultural soils is of increasing concern due to food safety issues and potential health risks. Various sources result in the accumulation of heavy metals in vegetables, soil being the major one. The consumption of vegetables containing these elements is one of the main ways in which they enter the human body. Vegetables are an important dietary source of trace elements like iron (Fe), manganese (Mn), copper (Cu), and zinc (Zn), which regulate human metabolism (Lokeshappa et al., 2012). While some elements have beneficial effects for humans, others like cadmium (Cd) are very toxic.
There are considerable differences among crops in their ability to accumulate and tolerate Cd (Bingham et al., 1975; Chizzola, 1994; Harmanescu et al., 2011; He and Singh, 1994). Consequently, identifying cultivars that accumulate low levels of Cd is of interest, especially for cultivation in soils prone to Cd accumulation. In addition, members of the Solanaceae, Chenopodiaceae, Cruciferae, and Asteraceae are considered to be robust accumulators of Cd (Kuboi et al., 1986). Fruit vegetables, such as the tomato (Solanum lycopersicum L.), pepper (C. annuum L.), and eggplant (Solanum melongena L.), are characterized by rather low rates of heavy metal translocation to the fruit (Angelova et al., 2009). The impact of grafting on the uptake of heavy metals by fruit vegetables has not been well investigated. One of the few reports documents a reduction in Cd concentration in eggplant fruit after grafting onto Solanum torvum Sw. (Arao et al., 2008). In particular, grafting S. melongena plants onto S. torvum reduced the Cd concentration in leaves and the stem by 67%–73% relative to self-grafting or grafting onto Solanum integrifolium Poir., in both Cd-polluted and unpolluted soils. The Cd concentration in xylem sap collected from the stems of S. torvum was 22% less than that in stems of S. melongena, indicating an appreciable restriction of Cd translocation from roots to shoots in the former. The Cd concentration in the roots of S. melongena and S. torvum were similar when plants were exposed to identical external levels of Cd (Mori et al., 2009). There are no studies documenting the use of grafting in peppers (C. annuum L.), or to reduce heavy metal concentration in the fruits.
Since peppers are able to accumulate Cd in sufficient amounts to be of public health concern (Lund et al., 1981), it is very important to reduce their Cd content when grown in Cd-contaminated soils. In this study, 52 Japanese pepper cultivars were screened for low levels of Cd in edible fruits. Cd-accumulating rootstocks were selected, and grafting to reduce the Cd concentration in fresh pepper fruits was tested.
Three experiments were carried out at the National Institute of Vegetable and Tea Science (Tsu, Japan) in 2008 and 2009. For all experiments, a gravelly gray lowland soil with organic matter content of 3.3%, total nitrogen content of 0.22%, pH 6.23, and 0.1 mol·L−1 HCl extractable Cd, Fe, Zn, Mn, and Cu of 2.1, 390.0, 102.5, 129.4, and 33.9 mg·kg−1, respectively, was used. The soil was collected from near an inactivated mine located in a mountainous region of south Japan. All plants received the same basal amount of N:P2O5:K2O as 0.25-0.25-0.25 g·kg−1 dry soil, in the three experiments. For all experiments, the soil moisture was maintained at around of 60% of field capacity by weighing the pots during cultivation.
Experiment 1: Screening Japanese peppers cultivars for low Cd in edible fruitsPeppers were grown in a 2.1 mg Cd·kg−1 naturally contaminated soil. The seeds were sown in small plastic pots containing 200 g of Cd-contaminated soil on April 1, 2008, and transplanted to the field on May 15, 2008. Plants were grown for 6 months and fruits were collected prior to the assessment of heavy metal concentration.
Experiment 2: Screening Japanese pepper rootstocks for low Cd absorptionEight pepper rootstock cultivars were grown in Cd-contaminated soil for 30 days. The seeds of rootstock cultivars were sown in small plastic pots containing 200 g of Cd-contaminated soil in April 1, 2008, and grown for 30 days prior to harvest of the aerial part (stems and leaves). The lowest Cd-accumulating rootstock was selected by measuring the Cd content of the aerial part of the seedlings.
Experiment 3: Grafting as a tool to reduce Cd in pepper fruitsScion and rootstock cultivars selected from experiments 1 and 2 were used. Among the Japanese pepper cultivars, the sweet cultivar that accumulated the highest amount of Cd in fruits selected in experiment 1 was used as the scion, while the cultivar that accumulated the lowest amount of Cd selected in experiment 2 was used as the rootstock. Non-grafted plants served as the control. The seeds of the scion and rootstock were sown in small plastic pots containing 200 g of Cd-contaminated soil on April 15, 2009, and grafted on May 23, 2009, using the splice grafting method.
Healthy, uniform seedlings of scion and root stock with 2–4 true leaves and a minimum stem diameter of about 1.5 mm to 2.5 mm were chosen for grafting. Rootstock and scion seedlings with a similar stem diameter were grafted and then placed in an incubator at 90% humidity for one week.
SamplingFor experiment 1, one fruit per plant was harvested when it reached a commercial size and/or color. For experiment 2, only the shoot (stems and leaves) was harvested for prior chemical analysis. For experiment 3, five fruits per plant were harvested when they reached a commercial size (30 g/fruit for the cultivar selected from experiments 1) and a fully ripe stage based on fruit color for chemical analysis.
Chemical analysesSoil samples, which were collected before pepper cultivation for chemical analyses, were air-dried then passed through a 2 mm sieve prior to analysis. Soil pH was measured in a soil suspension using a 1:2.5 (w/v) ratio of soil:deionized water. Cd, Fe, Zn, Mn, and Cu in soil were extracted using 0.1 mol·L−1 HCl (Nelson et al., 1959), extraction as stipulated by the Agricultural Land–Soil Pollution Prevention Law in Japan. Leaf color, as a SPAD value, was analyzed using a hand-held optical sensor (SPAD-502; Konica Minolta Inc., Tokyo, Japan) after flowering time. The SPAD values were measured at the center of the youngest fully-expanded leaf in each plant. The total organic C and N in the soil samples were measured using an automatic NC analyzer (Sumigraph NC22F; Sumika Chemical Analysis Service, Tokyo, Japan). The total organic matter was calculated by multiplying the total organic C by 1.72 according to Nelson and Sommers (1996).
Heavy metal (Cd, Fe, Zn, Mn, and Cu) content was measured as following: samples of harvested leaves, stems, and fruits were dried in an oven at 70°C for 48 h. Then, the samples were ground and digested in 5 mL HNO3 overnight in a digestion block (SCP Science Digi-PREP, Tokyo, Japan) with a strict temperature regime (30 min heating, 60 min at 70°C, 30 min heating, 120 min at 107°C), then left to cool overnight. Approximately 1 mL of H2O2 was added and the mixture was heated to 70°C. The entire process was repeated until the extract became clear.
The heavy metal concentration on the pepper fruits was expressed on a fresh weight basis. The concentration on a fresh weight basis was calculated from the water content of the sample and the mineral concentrations on a dry basis as follows:
The concentrations of heavy metals in the soil and plant extracts were measured by Inductively Coupled Plasma—Atomic Emission Spectroscopy (ICPS-1000IV; Shimadzu, Kyoto, Japan). Table 1 shows the wavelength, limit of detection (LOD), and limit of quantification (LOQ) for each element in the ICP-OES analysis.

Wavelength, limit of detection (LOD), and limit of quantification (LOQ) for each element in the ICP-OES analysis.
The experiments were conducted with a completely randomized design. For Experiment 1, 52 treatments (pepper cultivars) with 3 replications were made. For Experiment 2, eight treatments (rootstocks cultivars) and 3 replications, and for Experiment 3 two treatments (self-rooted and grafting) and 5 replications were performed. Treatment means were separated by two-way ANOVA and significant differences were assessed by post-hoc comparisons of means (LSD test, at P < 0.05).
The Cd, Fe, Zn, Mn, and Cu contents of fresh pepper fruits collected from plants after growing in soil highly contaminated with Cd are shown in Table 2. As shown in Figure 1, half of the cultivars had a Cd content higher than 0.05 mg Cd·kg−1, which is the maximum permitted level (MPL) established by the Codex Alimentarius Commission of the United Nations Food and Agriculture Organization (FAO) and the World Health Organization (WHO) for fruiting vegetables. Among the chili pepper cultivars, ‘Genki Amanaga’ (cv. 1) and ‘Manganji’ (cv. 52) contained the highest and lowest levels of Cd in the fruits (0.088 and 0.018 mg Cd·kg−1, respectively). Among the sweet pepper cultivars, ‘Suiko Shishito’ (cv. 2) and ‘Heian eiko’ (cv. 51) showed the highest and lowest Cd in the edible fruits (0.084 and 0.025 mg Cd·kg−1, respectively).

Trace element contents of fresh peppers from plants grown in a highly Cd contaminated soil.

Cadmium content of fresh pepper fruits of 52 Japanese varieties after growing in a highly Cd contaminated soil. Codex standard is the minimum level of Cd permitted in pepper fresh fruits established by the CODEX Alimentarius Commission of the United Nations Food and Agriculture Organization (FAO) and the World Health Organization (WHO) for fruiting vegetables. Each individual bar in the graph represents the standard error (SE).
As shown in Table 2, the concentration of trace elements in the fruits of the studied pepper cultivars ranged from 2.4 to 7.7 mg Fe·kg−1 FW, 2.0 to 8.5 mg Zn·kg−1 FW, 0.6 to 2.7 mg Mn·kg−1 FW, and 1.1 to 2.9 mg Cu·kg−1 FW. Among the sweet peppers, cultivar 2 contained the highest levels of Fe and Zn in the fruits. The ‘Ace’ (cv. 3) contained the highest level of 1.3 mg Mn·kg−1 FW and ‘Special’ (cv. 36) the highest level of 1.6 mg Cu·kg−1 FW. Among the chili pepper cultivars, ‘Takanotsume’ (cv. 10) contained the highest levels of Fe, Zn, Mn, and Cu of 7.7, 8.5, 2.7, and 2.9 mg·kg−1 FW, respectively, in the fruits. These values were significantly higher than those of the sweet pepper cultivars.
As shown in Table 3, a high correlation coefficient was observed among Fe, Mn, and Zn. On the contrary, an inconsistent correlation between Cd and the other elements was found. This probably implies that similar transport mechanisms control the translocation of Fe, Mn, and Zn to pepper fruits and that different transport mechanisms control the translocation of Cd to pepper fruits. An inconsistent correlation between Cd and Zn, Cu and Fe among leaves, stems, and roots of the tomato was reported (Dong et al., 2006).

Correlation coefficients of trace element contents in fresh fruits of Japanese pepper cultivars after growing in a highly Cd contaminated soil.
Table 4 shows the accumulation of Cd, Fe, Zn, Mn, and Cu in the aerial part (stems + leaves) of eight pepper rootstocks. Except for Mn, the ‘Daisuke’ rootstock showed the lowest accumulation of Cd, Fe, and Zn in the shoot. On the contrary, rootstock ‘Tougarashi Ano 5’ showed a high accumulation of all studied trace elements. ‘Daisuke’ rootstock was screened from the studied cultivars for use in the grafting experiment aimed at reducing the level of Cd in fresh peppers. Cultivar 3 was selected because it accumulated Cd in the fruit to a level that exceeded the CODEX index.

Accumulation of Cd, Fe, Zn, Mn, and Cu in shoots of Japanese pepper rootstocks after growing for 30 days in a Cd contaminated soil.
The effects of grafting cultivar 3 onto the ‘Daisuke’ rootstock on the Cd, Fe, Zn, Mn, and Cu concentration of pepper fruit are shown in Figures 2 and 3. This graft significantly reduced the Cd concentration in the fruits, but did not affect the levels of Fe, Mn, Zn, and Cu in the fresh fruits of grafted plants.
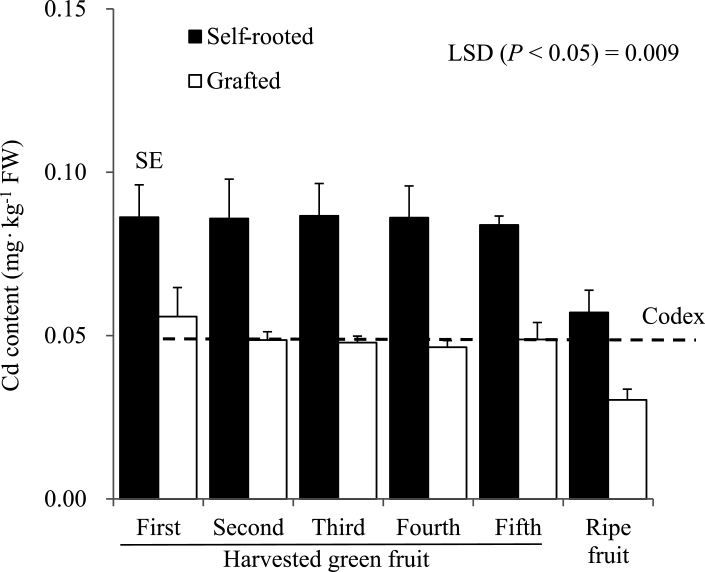
Effect of grafting of ‘Ace’ (cv. 2) onto the ‘Daisuke’ rootstock on Cd levels of peppers after growing in a contaminated soil. The pepper cv. 2 and the ‘Daisuke’ were used as the scion and rootstock, respectively. Codex = maximum level of Cd permitted in fresh peppers stablished by the CODEX Alimentarius Commission of the United Nations Food and Agriculture Organization (FAO) and the World Health Organization (WHO) for fruiting vegetables. Each individual bar in the graph represents the standard error (SE).

Effect of grafting of ‘Ace’ (cv. 2) onto the ‘Daisuke’ rootstock on (a) Fe, (b) Zn, (c) Mn, and (d) Cu levels of peppers after growing in a highly Cd contaminated soil. Each individual bar in the graph represents the standard error (SE).
Figures 2 and 3 show the effect of grafting of scion cultivar 3 onto the ‘Daisuke’ rootstock on Cd, Fe, Mn, Zn, and Cu levels in five fresh peppers harvested at the immature stage when they reached a commercial size (30 g for ‘cultivar 3’) and a fully ripe stage based on fruit color. The level of Cd in immature fresh fruits of grafted plants was reduced by 42% compared to self-rooted plants. The mean level of Cd in the first five harvested immature fruits was 0.086 mg·kg−1 for non-grafted plants and 0.05 mg·kg−1 for grafted plants.
The Cd in self-rooted fresh sweet peppers exceeded the Codex standard of 0.05 mg Cd·kg−1 permitted in the fresh weight of fruiting vegetables by the Codex Alimentarius Commission (Codex Alimentarius Commission, 2005). On the contrary, despite the high level of Cd contamination in the soil, the Cd concentration in fresh fruits decreased significantly in grafted plants. There are no studies in the literature regarding the content of Cd in fresh peppers or about the use of grafting to reduce Cd in pepper fruits.
As shown in Figure 2, it was clear that the rootstock affected the Cd content of fresh pepper fruits. The uptake mechanism and translocation of Cd by pepper plants is not well understood. Similar distribution patterns of Cd and Zn in the fruits of grafted peppers suggest that some common transport mechanism may exist for these elements in grafted plants. Some reports have shown a similarity between Cd and Zn transport in other vegetables. Zn and Cd are translocated from roots to shoots by P-type ATPase AtHMA4 in the roots of Arabdopsis thaliana (Verret et al., 2004). Cd and Zn are translocated across the tonoplast by a proton antiport (Gonzalez et al., 1999). The effect of Zn on Cd absorption by plants is of concern due to the similarity of Zn with Cd (Abdel-Sabour et al., 1988; Chaney et al., 1976; Cosio et al., 2004; Lombi et al., 2001; Nan et al., 2002; Oliver et al., 1994; Welch et al., 1999).
As shown in Figure 4 no visual differences between self-rooted and grafted plants were found. Table 5 shows some growth parameters of self-rooted and grafted pepper plants. No significant (P < 0.05) differences between plants were found for total shoot dry weight, leaf color, plant height, plant width, yield, or number of fruits per plant. Pepper fruits can accumulate a high amount of Cd and tolerate a high concentration of Cd in the soil and plant tissues without a corresponding reduction in yield (Georgieva et al., 1977). Furthermore, pepper plants should not be cultivated even in soils with a low level of Cd contamination. The use of grafting could be a solution to this problem. In this study, the concentration of Cd in fresh fruits was significantly lower in grafted compared with non-grafted pepper plants. However, as shown in Figure 3, other trace elements such as Fe, Mn, and Zn, which are important for human nutrition, were also reduced.

View of self-rooted (left) and grafted (right) plants.

Growth parameters of self-rooted and grafted pepper plants.
Among the Japanese pepper rootstocks, ‘Daisuke’ was selected as a low Cd-accumulating rootstock. The scion cultivar ‘Ace’ grafted onto the ‘Daisuke’ rootstock reduced the Cd content of pepper fruits. However, micronutrients such as Fe, Mn, and Zn, which are important for human diet, were also reduced.