2017 Volume 86 Issue 1 Pages 113-120
2017 Volume 86 Issue 1 Pages 113-120
We investigated the relationship between shoot apex morphology and anthesis time in five cultivars of Chrysanthemum morifolium flowering in summer. Fluctuating weather conditions in nature make stable production of agricultural crops difficult. The timing of floral induction and flower formation are determined by environmental factors such as day length and temperature. Although genetic analyses using model plants have provided a lot of knowledge regarding the molecular mechanism of flowering, it is difficult to apply to agricultural plants growing in the field because of the inconstant weather and lack of molecular analysis tools of many farmers. A simple morphological marker that enables farmers to predict anthesis time is useful for stable production of field-grown crops. We examined the shoot apex morphology of five Chrysanthemum morifolium cultivars weekly. Measurement of the shoot apex diameter by image analysis software showed that the shoot apex enlargement can be separated two phases, early slow-growth and later rapid-growth phases. We defined the developmental stages of the shoot apex in C. morifolium and found that the diameter of the shoot apex and developmental stages had a proportional relation. Each cultivar had a different inclination in an approximation straight line in the later rapid-growth phase. Comparison of the shoot apex morphology and weather records revealed different responses to the ambient temperature among the cultivars. The data suggest that weekly observation of the shoot apex makes it possible to characterize the cultivar and can be used to predict anthesis time. This is useful as a simple morphological marker for stable production of C. morifolium grown outdoors.
Control of seasonal production of agricultural crops is one of the most important techniques for farmers because development of many plants depends on the ambient environment. Recent global warming has affected the growth duration and region of cultivation: farmers have to adapt to this situation by changing the timing of sowing and harvesting, or switching to other cultivars (Schiermeier, 2015). Temperature affects several developmental processes including floral induction in many plants (Balasubramanian et al., 2006; Nakano et al., 2013; Van Der Ploeg and Heuvelink, 2006).
Chrysanthemum morifolium Ramat., belonging to the Asteraceae family, is one of the most popular ornamental plants in the world. Most of the chrysanthemum cultivars are short day plants, that is, floral transition is induced by continuous long-night. This induction can be suppressed by night break, which is used to control the timing of floral induction (Hakuzan and Kooriyama, 2014). Although this technique is useful for farmers to produce crops in the intended season, it is energy-consuming and difficult to use for field-grown crops. Cultivars with relatively small apical receptacles and elongated lateral shoots, called “Kogiku”, are amongst the most popular types in Japan (Fig. 1), where they are used as cut flowers. In Japan, sales of these flowers is highest in early August, when they are used to ceremonially decorate family tombs in honour of ancestors. In order to meet demand, farmers growing C. morifolium must be able to control the anthesis time. It has been found that high temperatures cause retardation of floral induction in the chrysanthemum (Nakano et al., 2013); thus, recent global warming has made their stable production difficult.

Chrysanthemum morifolium cultivars used in this study. (A) ‘Akemi’. (B) ‘Okina-maru’. (C) ‘Shiro-yamate’. (D) ‘Beni-shikibu’. (E) ‘Hiroshima-beni’.
Many studies using the model plant Arabidopsis thaliana have revealed the molecular mechanism of floral induction. Plants sense environmental factors, mainly day length, and integrate these seasonal signals with the expression of the florigen gene, FLOWERING LOCUS T (FT) in leaves. The FT protein moves to the shoot apex, where it interacts with a basic leucine zipper (bZIP) transcription factor, FD, and activates genes involved in flower formation, such as APETALA1 (Amasino, 2010; Fornara et al., 2010). TERMINAL FLOWER 1 (TFL1), from the same gene family as FT, represses flower formation at the shoot apical meristem by maintaining its indeterminate state (McGarry and Ayre, 2012). This floral induction system is largely conserved both in long-day and short-day crops, including rice, barley, wheat, and legumes (Greenup et al., 2009; Shrestha et al., 2014; Taoka et al., 2013; Tsuji et al., 2011; Weller and Ortega, 2015). Three FT-like genes have been identified from a wild diploid chrysanthemum, C. seticuspe. Of these, CsFTL3 plays a central role in floral induction in response to a short-day photoperiod (Oda et al., 2012). Overexpression of CsFTL3 in A. thaliana and the C. morifolium cultivar ‘Jimba’ causes early flowering (Oda et al., 2012). Moreover, the Anti-florigenic FT/TFL1 family protein (AFT) in C. seticuspe inhibits the action of CsFTL3 by interacting with CsFDL, a homolog of FD, under long-day conditions (Higuchi et al., 2013), suggesting conserved regulation of floral induction in the chrysanthemum.
Although these studies on floral induction have increased understanding of the molecular mechanisms, most of them examined plants grown in laboratories under controlled conditions of day length and temperature. However, many farmers of C. morifolium cultivars in Japan grow plants in the field, where flower growth is affected by the climate. Most farmers do not use molecular biological tools to check gene expression, which can be a good marker to predict the timing of floral induction. Therefore, establishment of morphological markers to predict anthesis time will help farmers with stable production in the intended season.
We examined the morphology of the shoot apex and leaves after floral induction to establish morphological markers for anthesis time prediction of C. morifolium. Our results suggest that weekly observation of the shoot apex provides the characteristic features of response to the environment, enabling famers to predict the anthesis time of chrysanthemum cultivars.
Five C. morifolium cultivars, ‘Akemi’, ‘Okina-maru’, ‘Shiro-yamate’, ‘Beni-shikibu’, and ‘Hiroshima-beni’, preserved in the Kyoto Prefectural Agriculture, Forestry and Fisheries Technology Center, were used for this study (Fig. 1). Dates of cultivation steps in 2014 and 2015 are listed on Table 1. Vegetative shoot cuttings, grown on the university farm of Kyoto Prefectural University in Seika, Kyoto, were transplanted into plastic pots filled with vermiculite in March and cultivated in a greenhouse with natural daylight conditions for either 3 (in 2015) or 4 (in 2014) weeks. Rooted cuttings were transplanted outside on the university farm in early April. After two weeks, the apex of the main shoot was pinched off to induce lateral shoot growth. Extra shoots other than three well-grown ones were cut off after one month. For leaf morphogenesis, rooted cuttings grown in Kameoka, Kyoto, were transplanted to the same university field on 15 April, pinched off on 23 April, and extra shoots were cut off on 20 May in 2013. Weather data in Kyotanabe, a neighbour city of Seika, was collected from the website of the Japan Meteorological Agency (http://www.jma.go.jp/jma/indexe.html).

Dates for Chrysanthemum cultivation.
Six shoot apices of each cultivar were collected weekly, dissected and photographed under a S8AP0 stereomicroscope and EC3 digital camera (Leica microsystems, Germany). Shoot apical meristem (SAM) diameter was measured using Image J software (Research Services Branch, National Institute of Mental Health, USA). Leaves formed on one shoot were collected 5 July, 2013, scanned, and the perimeter and area were measured using Image J software.
In C. morifolium, as well as many other flowering plants, floral induction causes the shoot apex to stop producing leaves and start to develop floral primordia. During this transition, the shoot apex enlarges and becomes dome-shaped through increased cell number and size (Popham and Chan, 1952), and starts developing bracts and two types of florets, peripheral ray florets and central disc florets. We expect that this morphological change could allow us to predict the anthesis time of C. morifolium grown in outdoor fields. To test our hypothesis, we examined the morphology of shoot apices in five cultivars during floral transition. These cultivars have either yellow (‘Akemi’ and ‘Okina-maru’), white (‘Shiro-yamate’), or dark purplish-red petals (‘Beni-shikibu’ and ‘Hiroshima-beni’), all of which are popular cultivars in the market in Kyoto, Japan (Fig. 1).
The developmental stages of the shoot apex up to floret initiation have been previously defined (Cathey and Borthwick, 1957; Horridge and Cockshull, 1979). We added definitions of later floret stages so that we could distinguish the developmental status of the apex (Fig. 2; Table 2). Weekly measurements of the diameter of the shoot apex inside bracts showed that the shoot apex of all cultivars began to expand and become dome-shaped towards flowering (Fig. 2A–D). From this stage, bracts started to form instead of leaves (Fig. 2C–E), and floret primordia initiated from the periphery in a spiral pattern (Fig. 2E, F). The terminal floret initiated at the top of the shoot apical meristem at stage 8 (Fig. 2G) then each floret developed sepals, petals, and reproductive organs (Fig. 2H–L). In the five cultivars used in this study, the outermost floret developed into ray florets, forming large colored petals, whereas inner florets developed into disc florets (Fig. 2J–L).

Developmental stage of the shoot apex in ‘Shiro-yamate’. Several peripheral leaves and bracts were removed in A to F. Top view (A–F) and side view of a transverse section (G–L) of the shoot apex. L, leaf primordia; b, bract; rf, ray flower. Bars: A–F, 0.5 mm; G–L, 1 mm.

Developmental stages of reproductive apical meristems (After Cathey and Borthwick, 1957, and Horridge and Cockshull, 1979).
The relation between developmental stage and diameter of shoot apices showed that enlargement of the shoot apex can be separated into two phases. In the early phase up to stage 6, they enlarged slightly and after stage 7, they did so more rapidly (Fig. 3A1–E1). This feature was common to all five cultivars. Approximation lines after stage 7 indicates that the floral stage and diameter of shoot apices had a proportional relation (Fig. 3A2–E2). The inclination of each straight line in five cultivars varied, indicating a difference in shoot apex development among cultivars. For example, ‘Akemi’ had the highest score of inclination (1.0717), indicating that the shoot apex expanded rapidly. On the other hand, ‘Beni-shikibu’ had a low score (0.7724), indicating a slower growth rate compared to the other cultivars.
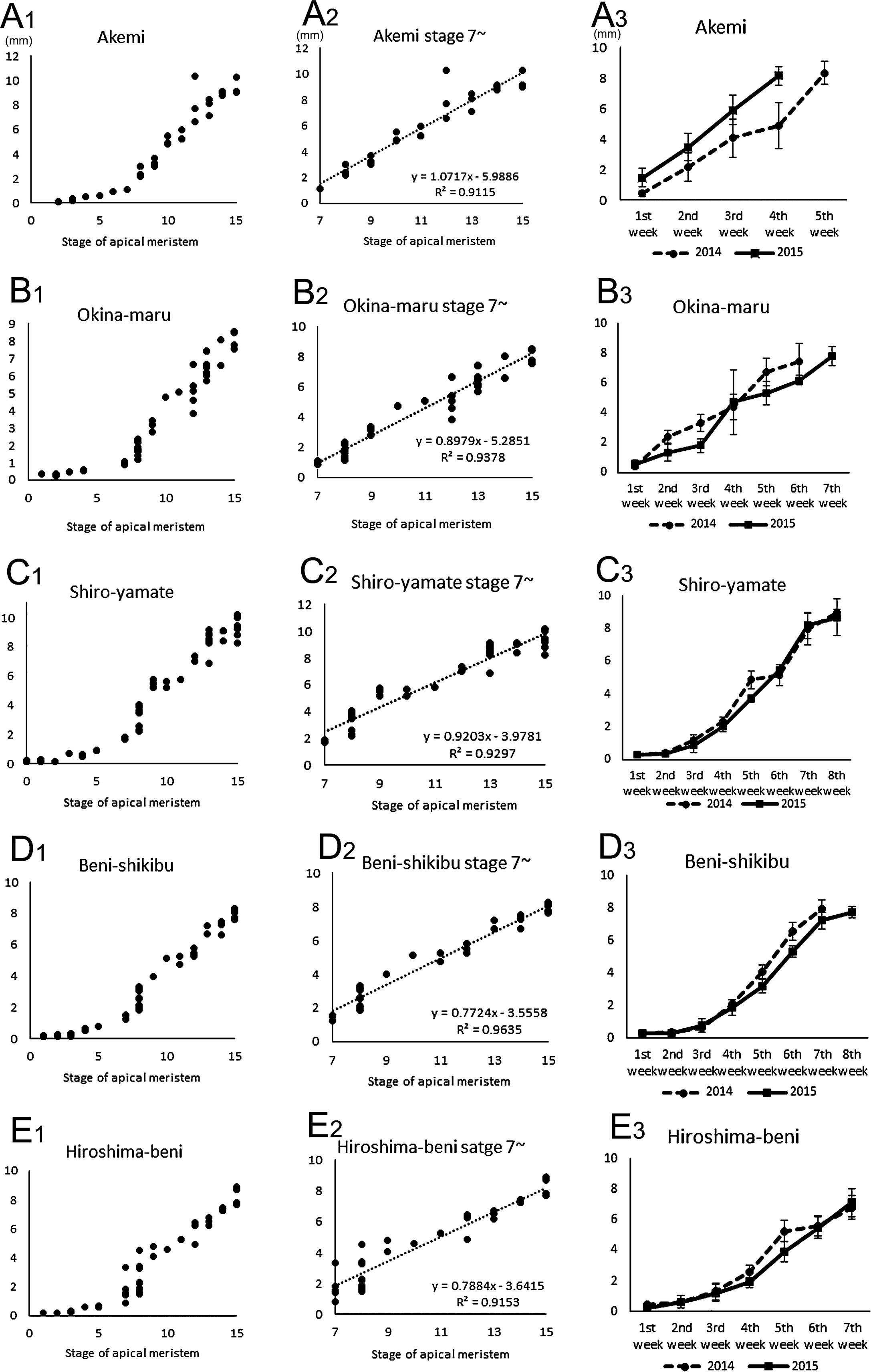
Diameter change during floral transition in five cultivars; (A) ‘Akemi’ (B) ‘Okina-maru’ (C) ‘Shiro-yamate’ (D) ‘Beni-shikibu’ (E) ‘Hiroshima-beni’. (A1–E1) Diameter and developmental stage. (A2–E2) Diameter change after stage 7. Dotted lines indicate approximation straight lines. Regression equation and coefficient of determination are shown. (A3–E3) Weekly change in the shoot apex diameter in 2014 and 2015. Six apices in each cultivar were examined every week.
To investigate whether variation in weather conditions affects shoot apex development, we compared the diameter in 2014 and 2015. In March 2015, transplanted shoots from the field were blighted, probably due to lower temperatures in this season so we transplanted again, causing a 10-day difference between 2014 and 2015 in terms of transplanting (Table 1). The other steps, such as transplanting of rooted cuttings to the field, pinching off of the shoot apex, and extra shoot cuttings were done on approximately the same dates in both years (Table 1).
In ‘Akemi’ and ‘Okina-maru’, the shoot apex expansion and anthesis dates in 2015 were 1-week later than in 2014 (Fig. 3A3, B3). This may reflect the 10-day difference in the first transplanting from the field, suggesting that their floral induction is not affected by ambient conditions, but rather by the internal developmental age. In contrast, ‘Shiro-yamate’ and ‘Hiroshima-beni’ developed their shoot apexes in almost the same manner both in 2014 and 2015 (Fig. 3C3, E3), indicating that their floral transition depends on day length. The shoot apex of ‘Beni-shikibu’ developed in the same manner up to the 4th week, but afterwards expanded slower in 2015 than in 2014 (Fig. 3D3), suggesting that shoot apex development is affected by ambient conditions in this cultivar. These data indicate that ‘Shiro-yamate’ and ‘Hiroshima-beni’ are less affected by the environment, so that they can be used for stable production, while the anthesis time of ‘Akemi’ and ‘Okina-maru’ can be controlled by transplanting date. The data also suggest that during cultivation, C. morifolium varieties have acquired different sensitivities to the environment which affect floral induction and shoot apex development.
In ‘Shiro-yamate’ and ‘Hiroshima-beni’, shoot apex enlargement was halted temporarily between the 5th and 6th week in 2014, but not in 2015 (Fig. 3C3, E3). Floral transition is delayed by high temperature in C. morifolium (Nakano et al., 2013), but after floral transition, it remains unknown whether the temperature affects shoot apex enlargement. We found that the temperature around one-week before this retardation was higher in 2014 than 2015 (Fig. 4). This suggests that high temperature affects the development of the shoot apex of chrysanthemums even after floral transition. Anthesis time of these cultivars was almost the same in the two years, despite developmental retardation, suggesting again that these cultivars are suitable for stable production.

Weather records in Kyotanabe in 2014 and 2015. (A) Temperature in June, July, and August in 2014 and 2015. (B) Precipitation in 2014 and 2015. (C) Hours of sunlight in 2014 and 2015. Duration when shoot apex development was retarded in ‘Shiro-yamate’ and ‘Hiroshima-beni’ in 2014 is squared.
Leaves on Chrysanthemum shoots change their shape depending on the plant age. Generally, leaves formed at an early phase (juvenile stage) have a simple shape, while those formed later tend to become more complex (Fig. 5A). Since leaves are flat, their shape is easily assessed by a scanner and image analysis software (Mokhtarian and Abbasi, 2004). We expected that this shape change can be a morphological marker to judge the plant age, enabling prediction of the anthesis time. To test this hypothesis, we quantified the leaf morphology of two cultivars ‘Shiro-yamate’ and ‘Beni-shikibu’ after floral transition in 2013. Because leaflets of one leaf were sometimes overlapped when scanned (Fig. 5A), we measured the perimeter and area of the top leaflet for each leaf (Fig. 5B). The perimeter of ‘Shiro-yamate’ and ‘Beni-shikibu’ became longer, up to 20th and 11th leaves, respectively (Fig. 5C, D), indicating that leaves formed at an early phase are juvenile, during which floral transition does not occur even under inducing conditions (Hong and Jackson, 2015). After that, the leaf perimeter of both cultivars reduced gradually, suggesting that they were developing. This tendency is the same in the area (Fig. 5E, F). These measurements suggest that leaves were more complex in ‘Beni-shikibu’ than ‘Shiro-yamate’. This is supported by the leaf dissection index (the ratio of the perimeter to the square root of the area), a parameter of leaf complexity (Fig. 5G, H: Kincaid and Schneider, 1983; Mclellan, 1993). Although leaf complexity can be quantified, it is difficult to use it for prediction of anthesis time. Rather, it can be used for cultivar characterization, because complexity of leaf morphology is also one of the factors related to cultivars.

(A) Morphology of 2nd (left) and 31st (right) leaves in ‘Shiro-yamate’. (B) Perimeter and surface area of the top lobe in scanned leaves were measured. (C, D) Perimeter of ‘Shiro-yamate’ (C) and ‘Beni-shikibu’ (D). (E, F) Area of ‘Shiro-yamate’ (E) and ‘Beni-shikibu’ (F). (G, H) Dissection index of ‘Shiro-yamate’ (G) and ‘Beni-shikibu’ (H). Bars: 1 cm. Leaves on three shoots in each cultivar were examined.
Establishing a simple morphological marker that enables farmers to predict the timing of agricultural production can reduce their labor needs and improve yields. We have shown that the diameter of the shoot apex and developmental stages have a proportional relation in C. morifolium grown outdoors, and that we can predict the near-future developmental status by weekly measurement of the shoot apex. This is a simple method compared to molecular analyses, so farmers can use this for stable production of C. morifolium in the intended season. This technique is also applicable to select suitable cultivars for regions with different weather conditions.
Ambient temperature impacts the time to flowering, number of flowers, and flower size in the Chrysanthemum (Van Der Ploeg and Heuvelink, 2006). Our data are from a 2-year investigation, but annual weather may change in the future, especially due to global warming, so that accumulation of annual data about shoot apex development and weather conditions will make this morphological marker more robust. Research on floral transition combined with the gene expression analysis of Arabidopsis plants in the wild showed a good correlation between floral transition and gene expression of FLOWERING LOCUS C and FT (Aikawa et al., 2010; Satake et al., 2013; Shimizu et al., 2011). A combination of observations of plant morphological changes, weather records, and molecular evidence can help farmers ensure stable production of agricultural plants grown outdoors.
The shoot apical meristem (SAM) is the central tissue to produce leaves and flowers in the vegetative and reproductive phases, respectively. In many flowering plants, the SAM enlarges upon floral transition (Hempel and Feldman, 1994). In indeterminate plants such as A. thaliana, the SAM keeps on bearing flowers at the flanks of the apex, and SAM per se maintains its undifferentiated state by positive feedback regulation mediated by WUSCHEL (WUS) and CLAVATA (CLV) proteins (Brand et al., 2000; Schoof et al., 2000). In flowers, which initiate as a floral meristem, WUS activity is repressed by AGAMOUS (AG) to lose the indeterminate features and form the gynoecium (Lenhard et al., 2001; Lohmann et al., 2001). The shoot apex of C. morifolium, a determinate plant, bears leaves at the flank of SAM during the vegetative phase: after floral transition, florets develop from the SAM periphery in a spiral pattern culminating in terminal floret formation at the apex (Fig. 2). Although the functions of WUS and CLV homologues have not been characterized in C. morifolium, downregulation of their function may correlate with the floral induction signal to acquire the determinate state.
Several studies have suggested techniques for regulating floral induction in C. morifolium. Gibberellin and cytokinin application can induce transition to the reproductive phase in non-inductive long day conditions (Pharis, 1972; Sumitomo et al., 2009). A mathematical model helps to predict the relation among apical dome mass and primordia initiation at the shoot apex during floral transition (Charles-Edwards et al., 1979). Molecular analyses have identified the genes involved in floral transition and floret initiation (Higuchi et al., 2013; Oda et al., 2012; Shchennikova et al., 2004). Unification of these physiological, mathematical, and genetic data, together with our morphological analyses, will reinforce the agricultural techniques for stable production of C. morifolium.
We thank Seisuke Kimura (Kyoto Sangyo University) for help in leaf morphological analysis, the staff in Kyoto Prefectural University agricultural field for helping cultivation, and Rebecca Horn (U.K.) for critical reading of the manuscript.