2017 Volume 86 Issue 3 Pages 327-333
2017 Volume 86 Issue 3 Pages 327-333
Calcium (Ca2+) concentration, early fruit growth, and expression of Ca2+-movement-related genes were analyzed during early fruit development in the tomato, which is the most important stage regarding the incidence of blossom-end rot (BER), to investigate the physiological mechanisms affecting the occurrence of BER. We used tomato introgression line IL8-3 with a chromosome segment from a wild relative (Solanum pennellii) because this line shows lower incidence of BER compared with the parent cultivar ‘M82’ (S. lycopersicum), as described previously. Ca2+ concentration in fruit and leaves was higher in IL8-3 than in ‘M82’, whereas no significant differences were observed between Ca2+ concentration in roots and stems of ‘M82’ and IL8-3. These results suggested that a Ca2+ transport property is an essential factor for the lower incidence of BER in IL8-3. IL8-3 fruit showed a lower growth rate than ‘M82’, which could result in preventing the occurrence of BER. The expression of genes encoding cation exchangers, Ca2+-ATPases, a Ca2+ channel, and Na+/Ca2+ exchangers, was higher in IL8-3 fruit than in ‘M82’ fruit, suggesting active Ca2+ movement in IL8-3. All results in this study could be related to physiological mechanisms accounting for the lower incidence of BER in IL8-3.
Calcium (Ca2+) plays important roles in plant growth and development because it maintains the integrity of the plasma membrane and the structure of the cell wall, and is involved in intracellular signaling (Hepler, 2005; White and Broadley, 2003). Thus, Ca2+ deficiency induces many kinds of physiological disorders, resulting in severe economic and productivity losses. The tomato (Solanum lycopersicum) is one of the most important vegetables due to its high economic and nutritional value and is also important as an experimental model species of the Solanaceae family and fleshy-fruited plants (Giovannoni, 2004; Shirasawa and Hirakawa, 2013). The tomato is sensitive to Ca2+ deficiency, which causes several physiological disorders in fruits such as cracking and blossom-end rot (BER) (White and Broadley, 2003). In particular, BER exhibits necrosis and discoloring in the distal portion of the tomato fruit and causes serious economic losses in tomato production (Taylor and Locascio, 2004).
Initial BER symptoms of tomato fruits are membrane leakage of cell solutes, cell plasmolysis, and membrane breakdown (de Freitas et al., 2011; Ho and White, 2005; Saure, 2001). Subsequently, the fruit surface exhibits symptoms of water soaking, and the tissue of the distal portion of the fruit becomes discolored and necrotic. BER is believed to be a Ca2+ deficiency disorder in tomato fruit because BER is induced by low Ca2+ content in fruit, and is inhibited after spraying Ca2+ on plants (Ho and White, 2005; Ho et al., 1993; White and Broadley, 2003). Apoplastic Ca2+ is necessary for appropriate plasma membrane integrity (Clarkson and Hanson, 1980; de Freitas et al., 2012; Hirschi, 2004; Kirkby and Pilbeam, 1984). Therefore, apoplastic free Ca2+ is maintained at a certain threshold to prevent membrane breakdown and damage (de Freitas et al., 2012; Kirkby and Pilbeam, 1984; Picchioni et al., 1998). The risk of BER occurrence becomes critical when Ca2+ in the distal portion of the immature fruit decreases to less than 0.20 μmol·g−1 fresh weight (Yoshida et al., 2014). Moreover, Ca2+-movement-related genes and Ca2+ concentration have been shown to be important in the incidence of BER in transgenic studies with their genes (de Freitas et al., 2011; Park et al., 2005). According to these previous studies, fruit Ca2+ concentration and Ca2+-movement-related genes, which may control Ca2+ accumulation and apoplastic Ca2+ levels, should be investigated in relation to BER.
BER is induced within 15 days after flowering (DAF) when fruit growth is most rapid (de Freitas et al., 2011; Saure, 2001). Even if sufficient Ca2+ is present in the whole fruit, an insufficient Ca2+ supply to cells in rapidly growing tissue may cause BER (Ho and White, 2005). High irradiance, temperature, and gibberellin, which promote early fruit growth, could result in the induction of BER (Ho et al., 1993; Saure, 2014). Therefore, it was also important to consider fruit growth within 15 DAF in this study.
Ho and White (2005) reported that plum tomatoes are more susceptible to BER than round tomatoes, and BER is never observed in cherry tomatoes and wild species. Therefore, cherry tomatoes and wild species may have genes that inhibit BER. However, evaluation of the agriculturally important traits in wild species is difficult because most of the useful traits are quantitative and some genes of wild species cause negative effects in modern cultivars (Ikeda and Kanayama, 2015). To facilitate the use and analysis of genes from wild tomato species, 76 introgression lines (IL) that contain a S. pennellii LA716 chromosome segment in the background of the cultivated plum tomato S. lycopersicum ‘M82’ were developed (Eshed and Zamir, 1994; Eshed et al., 1992). Solanum pennellii, a wild species of tomato, produces small green fruit and has genes that are related to stress tolerance (Bolger et al., 2014). These 76 ILs cover the entire genome and make it possible to evaluate individual quantitative trait loci (QTLs) (Lippman et al., 2007). We previously reported that IL8-3, one of the ILs that carries a S. pennellii chromosome segment on chromosome 8 of ‘M82’, shows a lower incidence of BER than ‘M82’ (Uozumi et al., 2012).
To the best of our knowledge, no reports have been published using ILs containing chromosome segments from wild relatives in relation to tomato BER except our previous work (Uozumi et al., 2012). Therefore, in the present study, Ca2+ concentration, early fruit growth, and expression of Ca2+-movement-related genes were analyzed in IL8-3 to investigate the physiological mechanisms affecting the incidence of BER.
The S. pennellii introgression line IL8-3 (Gur and Zamir, 2004) and the S. lycopersicum parent ‘M82’ were used in this study. ‘M82’ and IL8-3 were grown in a greenhouse using vermiculite and perlite supplied with half-strength Hoagland–Arnon nutrient solution as described in Uozumi et al. (2012) and their Ca2+ contents were determined. The plants used for the other experiments were grown in a greenhouse using culture soil:compost containing slow-release fertilizer and granular dolomite as described in Uozumi et al. (2012). Cultivation was carried out from April or May in a greenhouse under natural daylight. Temperature was maintained at above 19°C by heating when needed. Flowers were self-pollinated by hand without using plant growth regulators.
Determination of Ca2+ contentTo determine the Ca2+ content, fruits were harvested at 11–15 DAF, and roots, stems, and leaves were sampled just before the flowering period. All samples were dried at 80°C for three days and digested with nitric acid and perchloric acid to determine Ca2+ content using a Calcium Assay Kit LS (CPZIII) (Metallogenics, Chiba, Japan) and a microplate reader SH-9000 (Corona Electric, Ibaraki, Japan).
Fruit growth rate during early fruit developmentThe long and short diameter and height of fruits from 5 DAF to 15 DAF were measured everyday with calipers, and the increase per day was calculated from these data as described in Zheng et al. (1990).
Expression analysis of Ca2+-movement-related genesTotal RNA was extracted from the pericarp tissue of 10 DAF fruit by the SDS–phenol method (Mohammed et al., 2012). After removing genomic DNA from the RNA sample, quantitative reverse transcription (RT)-PCR was performed using the method of Hori et al. (2011) and Nishio et al. (2010) with the following program; 94°C for 15 min; 42 cycles of 94°C for 15 s, 56.1 or 58.3°C (Table 1) for 30 s and 72°C for 30 s. A search was made for Ca2+-movement-related genes in the tomato in the SOL genomics network (https://solgenomics.net/) on the basis of annotations of ITAG2.3 made by the International Tomato Annotation Group (ITAG) (Bombarely et al., 2011; The Tomato Genome Consortium, 2012). The gene-specific primer sets were designed using Primer 3 (http://primer3.ut.ee/), and the primer sequence and description of each gene are given in Table 1. Some primer sets were from de Freitas et al. (2011), and primer sets for SlActin as a control were from Mohammed et al. (2012).

Gene names, annotations, and primer sequences for quantitative RT-PCR in this study.
Significant differences were observed in Ca2+ content in 11–15 DAF fruits and leaves from ‘M82’ and IL8-3 plants (Fig. 1). The Ca2+ content in fruit and leaves in IL8-3 was approximately 16% and 45% higher than in ‘M82’, respectively, whereas no significant differences were observed between ‘M82’ and IL8-3 in roots and stems (Fig. 1).

Ca2+ content in fruits (A, 11–15 DAF), roots (B), stems (C), and leaves (D) of ‘M82’ and IL8-3. Values indicate means ± SE (n = 3). Significant differences between ‘M82’ and IL8-3 at P < 0.01 and 0.05, calculated using the t-test, are indicated by * and **, respectively.
Mean fruit diameter and height were measured from 5 to 15 DAF, and their increases per day are shown as fruit growth rate in Figure 2. Both fruit diameter and height in ‘M82’ were higher from 10 to 14 DAF than those in IL8-3, and significant differences were observed between ‘M82’ and IL8-3 at approximately 11 DAF.

Fruit growth rate during early fruit development in ‘M82’ and IL8-3. Long diameter (A), short diameter (B), and height (C) of 5–15 DAF fruits were measured daily with calipers. Each value indicates an increase (mm) from the day before. The average numbers of fruits were 9.8 and 9.3 in ‘M82’ and IL8-3, respectively. Values indicate means ± SE (n ≥ 3). Significant differences between ‘M82’ and IL8-3 at P < 0.05, calculated using the t-test, are indicated by *.
Forty-four genes were found as Ca2+-movement-related genes in the tomato from ITAG2.3 annotations, and 14 were selected as genes that are expressed differentially between ‘M82’ and IL8-3 in 10 DAF fruits based on our transcriptome analysis data (Ikeda et al., 2016). Genes used for gene expression analysis at 10 DAF were cation exchanger (CAX), Ca2+-ATPase, Ca2+ channel, and Na+/Ca2+ exchanger (NCX). Four CAX genes were analyzed and all of them showed higher expression in IL8-3 than in ‘M82’ (Fig. 3). In the four genes, CAX4 (Solyc09g005260) showed higher expression compared with the other three genes. The expression levels of six Ca2+-ATPase genes in IL8-3 fruit were higher than those in ‘M82’, and three of them showed significant differences between ‘M82’ and IL8-3 (Fig. 4). Among the six genes, Ca2+-ATPase3 showed higher expression compared with the other five. The expression levels of Ca2+-channel and NCX genes in IL8-3 fruit were higher than those in ‘M82’, and all genes showed significant differences (Figs. 5 and 6). Among the three NCX genes, Solyc07g062700 had the highest expression level (Fig. 5). Although de Freitas et al. (2011) reported that CAX7 (Solyc12g014110) is a cation exchanger, we analyzed it as NCX based on the ITAG2.3 annotation. Because the Ca2+ deficiency and BER were assumed to be triggered between 11 and 15 DAF, the expression analyses of Ca2+-movement-related genes, potential factors leading to Ca2+ deficiency and BER, were performed at 10 DAF. This allowed us to compare the results with our previous microarray data (Ikeda et al., 2016), which was also collected at 10 DAF.

Relative levels of transcripts of cation exchanger (CAX) genes in 10 DAF fruits of ‘M82’ and IL8-3. CAX3 (A), CAX4 (B), CAX6 (C), and Solyc12g055750 (D) expression was determined in the fruit by quantitative RT-PCR, and normalized against SlActin expression. Values indicate means ± SE (n = 3). Significant differences between ‘M82’ and IL8-3 at P < 0.01 and 0.05, calculated using the t-test, are indicated by * and **, respectively.

Relative levels of transcripts of Ca2+-ATPase genes in 10 DAF fruits of ‘M82’ and IL8-3. Ca2+-ATPase2 (A), Ca2+-ATPase3 (B), Solyc04g016260 (C), Solyc07g0026740 (D), Solyc09g082870 (E), and Solyc10g079300 (F) expression was determined in the fruit by quantitative RT-PCR, and normalized against SlActin expression. Values indicate means ± SE (n = 3). Significant differences between ‘M82’ and IL8-3 at P < 0.01 and 0.05, calculated using the t-test, are indicated by * and **, respectively.

Relative levels of transcripts of the Ca2+-channel gene (Solyc07g053970) in 10 DAF fruits of ‘M82’ and IL8-3. Gene expression was determined in the fruit by quantitative RT-PCR, and normalized against SlActin expression. Values indicate means ± SE (n = 3). A significant difference between ‘M82’ and IL8-3 at P < 0.01, calculated using the t-test, is indicated by **.
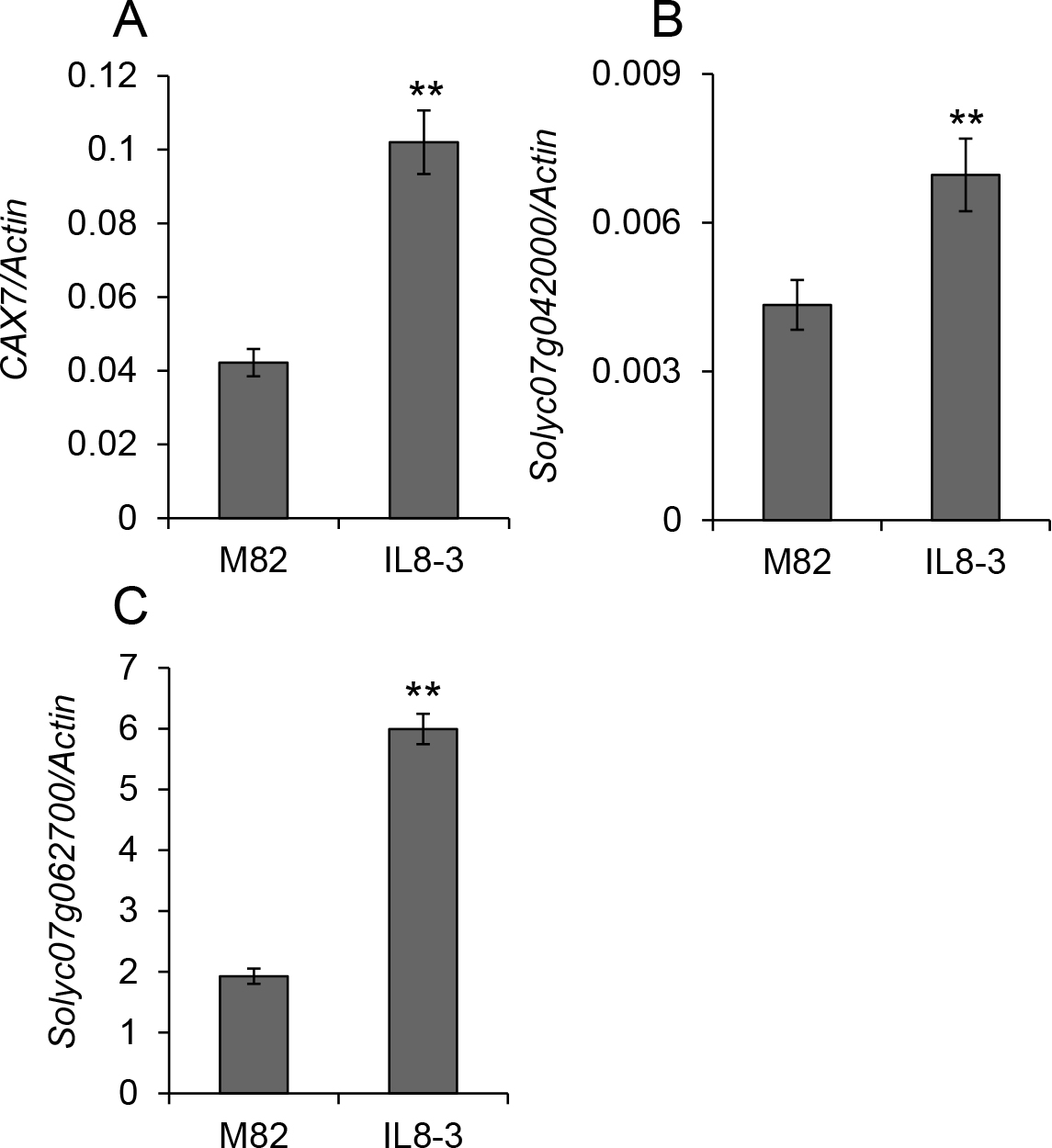
Relative levels of transcripts of Na+/Ca2+ exchanger (NCX) genes in 10 DAF fruits of ‘M82’ and IL8-3. CAX7 (A), Solyc07g042000 (B), and Solyc07g062700 (C) expression was determined in the fruit by quantitative RT-PCR, and normalized against SlActin expression. Values indicate means ± SE (n = 3). Significant differences between ‘M82’ and IL8-3 at P < 0.01 and 0.05, calculated using the t-test, are indicated by * and **, respectively.
We used fruits sampled between 11 and 15 DAF to determine Ca2+ content because BER is reported to be a symptom of a Ca2+-related disorder in tomato fruit that develops before 15 DAF. We also used vegetative organs in addition to fruits, in which Ca2+ content was already determined in IL8-3 (Uozumi et al., 2012). The results indicated that IL8-3 has the ability to accumulate more Ca2+ in fruit and leaves. Furthermore, the results suggest that a Ca2+ transport property is an essential factor for the lower incidence of BER in IL8-3.
Higher fruit growth rate during early fruit development and higher BER incidence were observed in ‘M82’ than in IL8-3, whereas the incidence in both genotypes was lower than that in the previous study (Uozumi et al., 2012). The lower BER incidence rate could be due to the somewhat early cultivation period. Therefore, the data for fruit growth rate in another year are shown in Figure 2 and, at that time, BER incidence was 52% and 17% in ‘M82’ (n = 7) and IL8-3 (n = 6), respectively (significant difference at P < 0.05, t-test). The early growth rate of fruit in ‘M82’ was found to be higher than that in IL8-3 in this study. Because a lower fruit growth rate could result in a decreased incidence of BER (Saure, 2001), the incidence of BER in IL8-3 fruit could be inhibited by a relatively low fruit growth rate. A previous study suggests the importance of fruit growth between 10 and 15 DAF from the observation of fruit diameter during fruit development in a single tomato genotype (Spurr, 1959), and the present study confirms this finding by comparing IL and its parent cultivar.
CAX transports Ca2+ mainly into the vacuole using the proton or sodium ion gradient (Cheng et al., 2005; Hirschi et al., 1996; Manohar et al., 2011; Shigaki et al., 2006). Ca2+-ATPases are mainly localized in the plasma membrane and actively transport Ca2+ to the apoplast against substantial concentration gradients in plant cells using ATP (Axelsen and Palmgren, 2001; White and Broadley, 2003). The Ca2+ channel and NCX are localized in the plasma membrane (Bano et al., 2005; White, 2000; White and Broadley, 2003). Information is lacking on the expression of these genes in tomato fruit and there are few reports on the relationship between their expression and BER. In the present study, there were large differences in expression levels among Ca2+-movement-related genes. Moreover, the expression of three Ca2+-ATPase genes (Solyc07g026740, Solyc09g082870, Solyc10g079300) and one NCX gene (Solyc07g042000) was very low in each gene family. Expression in nine of the ten remaining genes was higher in IL8-3 fruit than in ‘M82’ fruit at 10 DAF, which is the important stage for the occurrence of BER. In particular, CAX4, Ca2+-ATPase3, and Solyc07g062700 (NCX), for which expression was the highest among each gene family both in the present results of real-time PCR and in previous data from transcriptome analysis (Ikeda et al., 2016), showed higher expression in IL8-3 fruit. These results suggest that Ca2+ movement is more active in IL8-3 than in ‘M82’ fruit, and these three genes may play major roles in Ca2+ movement. The higher expression of CAX genes in IL8-3 could result in the lower incidence of BER because the introduction of the CAX gene increases Ca2+ content in tomato fruit (Park et al., 2005). The higher expression of Ca2+-ATPase genes could also be related to the lower incidence of BER because Ca2+-ATPase increases apoplastic Ca2+ levels, which are critical for the maintenance of membrane integrity (White and Broadley, 2003). Information about the expression of Ca2+-movement-related genes in leaves and the levels of apoplastic Ca2+ in fruit will be necessary to propose detailed mechanisms to explain the lower incidence of BER in IL8-3.
We previously reported that the key gene for BER is located within the restricted chromosome region of the IL8-3 region (Uozumi et al., 2012), and further genomic study has revealed that this region is approximately 610 kbp including 78 genes (ITAG2.4 and SOL genomics network). However, the Ca2+-movement-related genes analyzed here are located in other chromosomes than chromosome 8 (Table 1). At least one of three factors found in IL8-3 (high transport of Ca2+ to fruit; low fruit growth rate at the early stage; and high expression of Ca2+-movement-related genes) is likely regulated by one of the 78 genes to reduce the incidence of BER in IL8-3. One possible mechanism is that increased expression of CAX, for example CAX4, by a regulatory gene located in the region promotes Ca2+ accumulation in IL8-3 fruit and the resultant higher Ca2+ concentration positively controls the expression of other Ca2+-movement-related genes (Lee et al., 2013; Quiles-Pando et al., 2013), such as Ca2+-ATPase3 transporting Ca2+ to the apoplast, enhancing the level of apoplastic Ca2+. Fine mapping of this region will reveal the primary factor and a comprehensive mechanism.
The authors thank Ms. A. Uozumi for her help and the National BioResource Project tomato (NBRP tomato) for information.