2017 Volume 86 Issue 4 Pages 463-469
2017 Volume 86 Issue 4 Pages 463-469
Various on-tree ethanol-sticker treatments for ‘Taigetsu’ and ‘Taiten’ persimmon (astringent cultivars) were tested to identify optimal treatment conditions for astringency removal and fruit quality. Using aluminum foil coated or polyethylene terephthalate stickers to attach a pad containing 1.5 g of ethanol powder to both sides of the fruit surface in early October removed astringency completely, while the application of treatments to one side of the fruit surface did not. The astringency removal was incomplete in treatments with a cast coating sticker. The aluminum foil-coated sticker with a 1.0 g ethanol pad applied in early October removed flesh astringency completely without fruit skin darkening or the occurrence of large numbers of brown specks in the flesh. It extended fruit shelf life by approximately 7 days compared with postharvest CO2 treatment in November. Conversely, sticker treatments in late September caused many brown specks in the flesh. The sticker treatment with a 0.6 g ethanol pad failed to remove the astringency completely while the sticker treatment with a 1.5 g ethanol pad resulted in heavy fruit skin darkening or the appearance of shallow concentric cracks on the fruit skin. Therefore, we conclude that an on-tree sticker treatment with a 1.0 g ethanol pad on both sides of the fruit surface in early October removes fruit astringency completely and provides high quality ‘Taigetsu’ and ‘Taiten’ persimmon fruit.
Persimmon (Diospyros kaki Thunb.) are classified as either astringent or non-astringent at harvest. Currently, fruit from astringent cultivars are treated with ethanol, carbon dioxide (CO2) gas, or are dried to remove astringency before they are sold in Japan. The new Japanese persimmons ‘Taigetsu’ and ‘Taiten’ are both astringent-type cultivars bred from a cross between ‘Kurokuma’ and ‘Taishuu’ at the NARO Institute of Fruit Tree Science in Japan (Yamada et al., 2012a, b). ‘Taigetsu’ is a mid-ripening cultivar, with an average fruit weight of 450 g. While the flesh is soft and very juicy, shallow concentric cracks on the fruit skin often occur. ‘Taiten’ is a late-ripening cultivar, with an average fruit weight of 500 g. The flesh is soft and the fruit is of high eating quality, similar to that of ‘Taishuu’. The shelf life of ‘Taiten’ fruit after constant-temperature short-duration (CTSD) treatment with CO2 averaged 21 days at ambient temperature and was longer than that of ‘Hiratanenashi’ (Yamada et al., 2012b). However, the loss of astringency following CTSD treatment was slow (Yamada et al., 2012b), and the high temperatures used in this treatment caused a sour taste (Yamasaki et al., 2012). A more effective method of removing astringency and suppressing sourness is needed.
One such method is on-tree ethanol treatment, where fruit on the tree are enclosed in polyethylene bags with an astringency removal agent for 2 days at the beginning of peel coloration (Sugiura et al., 1975, 1977; Taira et al., 1987). When harvested, the astringency of the fruit is completely removed in ‘Hiratanenashi’. While the treated fruit remain firm and the eating quality stays good, skin darkening does occur and drying of the calyx can cause fruit drop. Bagging while excluding the calyx can reduce both fruit stain and fruit drop (Hirai et al., 2008). Treatment of ‘Taiten’ from mid-September until early October can completely remove astringency, and the fruit are of excellent eating quality (Miyata et al., 2011), but this is time consuming and labor intensive. To reduce labor, Takagi (2014) developed a method of attaching stickers directly to the fruit surface. Compared with CTSD treatment, this simple new method does not require large-scale facilities and advanced skills. However, the optimum conditions for on-tree sticker treatment of ‘Taigetsu’ and ‘Taiten’ persimmons remain unknown.
The objective of the present study was to determine the optimum conditions for on-tree sticker treatment in ‘Taigetsu’ and ‘Taiten’. We examined the effect of the material and position of the sticker, as well as ethanol amount in the pads and the timing of treatment to optimize on-tree sticker treatment.
Top-grafted nine- to 13-year-old Japanese persimmon ‘Taigetsu’ and ‘Taiten’ trees were used in this study. The trees were grown in an orchard at the Grape and Persimmon Research Division, NARO Institute of Fruit Tree and Tea Science, Hiroshima, Japan. Flower buds were thinned in mid-May to one bud per 13 leaves. Fruit were finally thinned to a ratio of 20 leaves to one fruit in mid-July.
Effect of the material and position of the stickerTo remove fruit astringency, we used stickers (110 mm × 120 mm) with three different materials and put an ethanol pad (Antimold Mild 15 containing 1.5 g of alcohol powder; Freund Industrial Co., Ltd., Tokyo, Japan) on each. The pad was made with ethanol-containing silica gel packed in a special film and laminated with ethylene-vinyl acetate and Japanese paper with very high gas permeability that regulates ethanol diffusion. Three types of material were examined: those with an aluminum foil coating (65 μm thickness), a PET (polyethylene terephthalate, 25 μm thickness), and a cast coating (92 μm thickness) (Maruu Secchaku Co., Ltd., Ehime, Japan) (Fig. 1A–C). These stickers were attached to either both sides of the fruit surface, one side of the fruit surface, or the fruit apex (Fig. 2) of individual fruits on the tree. In the treatment of both sides of the fruit surface and one side of the fruit surface, the ethanol pads were attached onto the equatorial area. ‘Taigetsu’ and ‘Taiten’ fruit (n = 3) selected randomly on the tree were subjected to each treatment on 2 October (127 days after full bloom, DAFB) in 2012, and the stickers were removed 2 days later (both sides of the fruit surface) or 3 days later (one side of the fruit surface and fruit apex).

Sticker material for on-tree sticker treatment. (A) Aluminum foil-coated sticker. (B) Polyethylene terephthalate sticker. (C) Cast-coated sticker. (D) On-tree ethanol treatment excluding the calyx.

Sticker position for on-tree sticker treatment. (A) Treatment of both sides of the fruit surface. (B) Treatment of one side of the fruit surface. (C) Treatment of the fruit apex.
Control ‘Taigetsu’ and ‘Taiten’ fruit were treated with CO2 gas; this has been one of the most popular commercial methods. At harvest, three fruit (n = 3) from each cultivar were placed in an incubator at 26°C for 16 h and then put into a 16.8-L plastic container. The internal gas in the container was replaced with 100% CO2 gas and the container sealed. After 24 h at 26°C, the container was opened. These fruit were then kept at 26°C in air for 6 days.
Treated ‘Taigetsu’ and ‘Taiten’ fruit were harvested at the fully colored stage in each cultivar on 6 and 19 November, respectively, and weighed. The skin color was assessed at the fruit apex against a ‘Fuyu’ skin-color chart index (Yamazaki and Suzuki, 1980). Flesh firmness was determined at two points on the cut surface horizontally with a fruit hardness tester (KM-5; Fujiwara Scientific Co., Ltd., Tokyo, Japan) fitted with a 5-mm cylindrical plunger. The degree of astringency was assessed using the tannin print method (Taira, 1996). The longitudinal cut surface of the fruit was pressed on 3% FeCl3-impregnated filter paper, and the degree of astringency was evaluated visually on a scale of 0 (non-astringent, −); 1 (almost not astringent, ±); 2 (slightly astringent, +); 3 (rather astringent, ++); and 4 (strongly astringent, +++); as described by Taira (1996). Values of ≤ 1 indicate that the fruit is edible.
Effect of ethanol pad content and the timing of treatmentThree types of ethanol pads (Antimold Mild 15, Antimold Mild 10, or Antimold Mild 6 containing 1.5, 1.0, and 0.6 g of alcohol powder, respectively; Freund Industrial Co., Ltd.) were attached to both sides of the equatorial fruit surface using aluminum foil-coated stickers. ‘Taigetsu’ and ‘Taiten’ fruits (n = 20) randomly selected on the tree were treated with each type of ethanol pad on 25 September (120 DAFB) or 2 October (127 DAFB) in 2013, and the stickers were removed 2 days later. Control fruit were treated with on-tree ethanol treatment excluding the calyx (control 1), and treated with CO2 gas at harvest as described above (control 2). For the on-tree ethanol treatment excluding the calyx, fruit before harvest were enclosed on the tree with a 0.03-mm-thick polyethylene bag containing an astringency removal agent (3 g, Sibutoru; Niitaka Co., Ltd., Osaka, Japan), sealed with a rubber band at the calyx (Fig. 1D). The timing of treatment was the same as that of the on-tree sticker treatment. The bags were removed after 2 days of treatment.
On-tree treated ‘Taigetsu’ and ‘Taiten’ fruit were harvested at the fully colored stage in each cultivar on 6 and 18 November, respectively. The fruit quality was assessed as described above and evaluated daily throughout the shelf life period. The soluble solids content in the flesh were measured with a digital refractometer (N-1; Atago, Tokyo, Japan). The degree of flesh darkening, where brown specks occur in the flesh tissue, was classified on a scale of 0 = no darkening, 1 = slight darkening, 2 = moderate darkening, and 3 = having very dark flesh. Ten fruits in each treatment were stored at 20°C, and softening was determined according to Iwata et al. (1969) who determined that a softened fruit had reached stage III of the softening index when it was soft enough to be easily crushed by the fingers, or when part of the fruit had a water-soaked appearance. Black staining on the fruit skin was assessed visually along with the occurrence of shallow concentric skin cracks and darkened fruit skin. The degree of black staining on the fruit skin was defined as being none (0%), little (30% or less), medium (30–70%), or high (70% or more).
Statistical analysisThe Bartlett test was used to examine the homogeneity of variance in the data. Analysis was performed using an ANOVA and Tukey-Kramer test in the case of equal variances, or a Kruskal-Wallis test and a Scheffe’s F test in the case of unequal variances. The comparisons between the flesh darkening were analyzed by two-way ANOVA.
Treating both sides of the fruit surface with the aluminum foil-coated and PET stickers completely removed astringency, with astringency scores ranging from 0.0 to 0.3 in both cultivars (Fig. 3; Table 1). When only one side of the fruit surface was treated with the aluminum foil-coated and PET stickers all fruit remained astringent, with astringency scores ranging from 1.0 to 2.0 in both cultivars. In the fruit treated at the apex with the aluminum foil-coated stickers, the astringency of ‘Taigetsu’ fruit was completely removed, but ‘Taiten’ fruit remained astringent. When the cast-coated stickers were used, all fruit remained very astringent, with astringency scores ranging from 2.3 to 4.0 in both cultivars. Conversely, treatment of the control fruit with CO2 completely removed astringency in all fruit, with an astringency score of 0.0.

Effect of sticker material and position on astringency of ‘Taigetsu’ and ‘Taiten’ persimmons. PET, polyethylene terephthalate. Vertical bars indicate SE (n = 3). Astringency score: 0 = non-astringent, 4 = strongly astringent.

Effect of the material and position of the sticker on removal of astringency and fruit quality of ‘Taigetsu’ and ‘Taiten’ persimmonsz.
In terms of fruit quality, extensive flesh darkening was observed on treated ‘Taiten’ fruit, but was absent in ‘Taigetsu’ fruit (data not shown). No significant differences were observed among treatments for fruit weight, skin color, or flesh firmness in ‘Taigetsu’ (Table 1). There were significant differences in fruit weight, skin color, and flesh firmness in differently treated ‘Taiten’ fruit, though this was unaffected by the presence or absence of astringency. Flesh firmness of fruit treated on both sides of the fruit surface with the aluminum foil-coated stickers was higher than that of CO2-treated fruit from both cultivars.
Effect of ethanol pad content and the timing of treatment on removal of astringency and fruit qualityAll treatments, except the 0.6 g/early October treatment, resulted in complete removal of astringency, with an astringency score of 0.0 in both cultivars (Fig. 4; Table 2). In the 0.6 g/early October treatment, it was observed using the tannin print method that astringency remained in the flesh near the calyx. Control fruit treated with CO2 gas were slightly astringent with an astringency score of 0.4 in ‘Taigetsu’ and 0.2 in ‘Taiten’.

Effect of ethanol pad content and the timing of on-tree sticker treatment on the astringency of ‘Taigetsu’ and ‘Taiten’ persimmons. Vertical bars indicate SE (n = 20). Astringency score: 0 = non-astringent, 4 = strongly astringent.

Effect of ethanol pad content and the timing of on-tree sticker treatment on removal of astringency and fruit quality of ‘Taigetsu’ and ‘Taiten’ persimmonsz.
In terms of fruit quality, flesh darkening was very slight or absent in the early October treatment, but significantly darker in the late September treatment for both cultivars (Table 3; Fig. 5). No significant differences were observed among treatments for skin color and soluble solids content (SSC) in ‘Taigetsu’ and fruit weight in ‘Taiten’ (Table 2). There were significant differences in fruit weight in ‘Taigetsu’ and skin color and SSC in ‘Taiten’, but no differences among the treatments for these traits by Tukey-Kramer test or Scheffe’s F test. The flesh firmness of sticker-treated ‘Taiten’ fruit was significantly higher than that of CO2-treated fruit (Table 2). There was no difference in flesh firmness in ‘Taigetsu’, but sticker-treated fruit had a crispy texture compared with CO2 treatment.
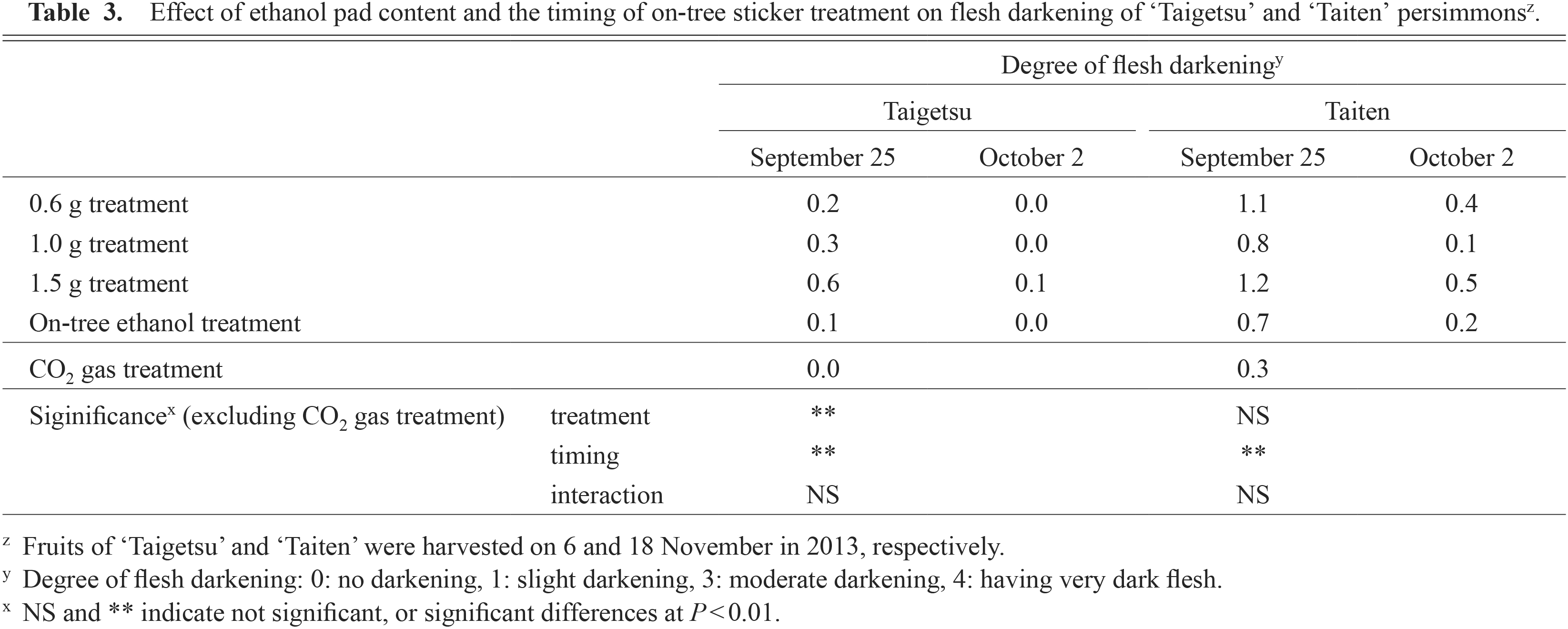
Effect of ethanol pad content and the timing of on-tree sticker treatment on flesh darkening of ‘Taigetsu’ and ‘Taiten’ persimmonsz.

Degree of flesh darkening in ‘Taigetsu’ and ‘Taiten’ persimmons.
With regard to storability, the shelf life of sticker-treated fruit was 18.9–20.7 days in ‘Taigetsu’ and 21.7–23.0 days in ‘Taiten’ (Table 2). The shelf life of CO2-treated fruit was 11.9 days in ‘Taigetsu’ and 15.3 days in ‘Taiten’. On-tree sticker treatment of fruit extended their shelf life by approximately 7 days relative to CO2-treated fruit.
Shallow concentric cracks appeared on the skin of ‘Taigetsu’ fruit in all treatments at harvest, especially with the 1.5 g ethanol pad treatment and the on-tree ethanol treatment excluding the calyx (Table 4). Conversely, ‘Taiten’ fruit developed darkened fruit skin, a physiological disorder, particularly with the same 1.5 g ethanol pad and with on-tree ethanol treatment excluding the calyx (Table 4).

Effect of ethanol pad content and the timing of on-tree sticker treatment on shallow concentric skin cracks or fruit skin darkening of ‘Taigetsu’ and ‘Taiten’ persimmonsz.
Treating both sides of the fruit surface of ‘Taigetsu’ and ‘Taiten’ fruit with the aluminum foil-coated and PET stickers completely removed fruit astringency (Fig. 3). However, our experiment showed when only one side of the fruit surface or the fruit apex was treated, the astringency was removed only on that side. This supports the result of the experiment where Miyata et al. (2011) enclosed only half of ‘Taiten’ fruit with polyethylene bags. In that experiment, they found astringency remained on the untreated side. Therefore, we assume that the large size of ‘Taigetsu’ and ‘Taiten’ fruit makes it difficult to remove the astringency with a single sticker in a short period of time. All fruit treated with the cast-coated stickers remained very astringent (Fig. 3). The cast-coated sticker is made of paper, and it is, therefore, possible that the ethanol vaporized from the ethanol pad and was released to the outside of the sticker rather than onto the fruit surface. As ‘Hiratanenashi’ fruit attached with PET remained astringent (Yamasaki et al., 2015), we selected stickers with the aluminum foil coating for the on-tree sticker treatment.
Fruit treated with 0.6 g ethanol pads in late September lost their astringency, but fruit treated in early October remained astringent near the calyx. Taira et al. (1990) showed that deastringency was greater with increasing concentrations of ethanol. Astringency could also be removed with difficulty using late on-tree ethanol treatments (Kataoka et al., 1993). In the early October treatment, the 0.6 g ethanol pad appeared to be insufficient to remove astringency, although the 1.0 and 1.5 g ethanol pads were sufficient for on-tree sticker treatment of both cultivars at this time. The use of the 1.5 g ethanol pad resulted in the occurrence of shallow concentric skin cracks or fruit skin darkening in ‘Taigetsu’ and ‘Taiten’ in the late September and early October treatments (Table 4). These shallow concentric skin cracks or fruit skin darkening were not observed in either cultivar with 1.0 g ethanol pad treatments. While Hirai et al. (2008) found a high level of stained skin in the mid-October treatment, our results show that treatment in the early or late stages did not influence the occurrence of black stains on fruit skin. This implies that the occurrence of black stains on the fruit skin is affected by ethanol concentration rather than the timing of treatment. We therefore conclude that a 1.0 g ethanol pad is the most suitable for on-tree sticker treatment in ‘Taigetsu’ and ‘Taiten’.
Flesh darkening occurred in late September, but not in early October-treated fruit (Table 3; Fig. 4). Sugiura et al. (1975, 1977) reported that flesh darkening occurred in fruit subjected to on-tree astringency removal and that the earlier ethanol treatment caused more extensive darkening. Hirai et al. (2008) found that flesh darkening occurred mostly with early treatment (September 20), but not with late treatment (October 12) in ‘Hiratanenashi’. Furthermore, treatment from 13 to 20 September (115 to 122 DAFB) resulted in strong flesh darkening in Gunma Prefecture, Japan. The timing of treatment in this study (120 DAFB) corresponded to the timing of a higher occurrence of flesh darkening in fruit as reported by Hirai et al. (2008). Flesh darkening may be caused by the accelerated polymerization of tannins by increasing polyphenol oxidase activity in early treated fruit (Sugiura et al., 1985). While some consumers prefer fruit with strong flesh darkening, flesh darkening spoils the taste and results in a coarse texture. Therefore, we suggest that on-tree sticker treatments should be done in early October. The flesh of sticker-treated ‘Taigetsu’ and ‘Taiten’ fruit were firmer than CO2-treated fruit and the shelf life improved by approximately 7 days when stored at 20°C (Table 2). CO2 gas treatment can also cause shallow concentric cracks in ‘Taigetsu’ fruit that are followed by accelerated fruit softening (Yamada et al., 2012a). On-tree sticker-treated fruit did not soften the fruit, and the shelf life was prolonged. Taken together, these results indicate that on-tree sticker treatment can remove astringency in ‘Taigetsu’ and ‘Taiten’ fruit and is effective for improving the incidence of physiological disorders that accompany on-tree ethanol treatment and CO2 gas treatment in both cultivars. The aluminum foil-coated stickers were, however, hard to remove because of their thickness, so further studies examining how to remove these stickers more easily are expected to improve the on-tree ethanol-sticker treatment method further.
In conclusion, on-tree sticker treatment with 1.0 g ethanol pads placed on both sides of the fruit surface using aluminum foil-coated stickers in early October removed ‘Taigetsu’ and ‘Taiten’ fruit astringency. Given the reduced level of flesh darkening and black skin staining relative to other treatments, and the resulting firm fruit with excellent eating quality, this was found to be an optimal deastringency method for producing high-quality fruit in ‘Taigetsu’ and ‘Taiten’.
We thank Dr. Akihiko Sato, Dr. Atsushi Kono, and Dr. Noriyuki Onoue at NARO Institute of Fruit Tree and Tea Science for kindly providing the experimental materials. We also thank Ms. Toyoko Morishige and Ms. Naho Takahashi for her support and technical assistance. Finally, we are grateful to research grant from the NARO Gender Equality Program.