2017 Volume 86 Issue 4 Pages 447-455
2017 Volume 86 Issue 4 Pages 447-455
Histological investigations of the fruit abscission zone and morphological changes in abscission zone cells in ponkan (C. reticulata Blanco), hyuganatsu (C. tamurana hort. ex Tanaka), ‘Kiyomi’ (C. unshiu × C. sinensis (L.) Osbeck), and satsuma mandarin (Citrus unshiu Marcow.) were conducted using detached fruits incubated in agar medium under 25°C, 4 weeks after anthesis during secondary physiological fruit drop. In the 96 h after the fruit abscission induction by detaching the fruits, the cumulative abscission ratio was 100% in ponkan, 22% in ‘Kiyomi’, and below 10% in hyuganatsu and satsuma mandarin. Fruit abscission began at 36 h in ponkan and ‘Kiyomi’, at 54 h in satsuma mandarin, and at 60 h in hyuganatsu after the fruit detachment. The fruit abscission zone was located on the connected part between the fruit and the disc in ponkan and hyuganatsu on the disc tissue in satsuma mandarin and ‘Kiyomi’. During the fruit abscission process, no abscission layer was observed at the abscission zone in these species and cultivars. Morphological changes in the abscission zone cells were determined by scoring cell changes (a score from 0 to 4) at five positions of the abscission zone. In ponkan, the morphological changes in the abscission zone cells, which began 30 h after fruit abscission induction, were synchronized in a symmetrical position in the abscission zone. The changes in ‘Kiyomi’ began at 30 h, and they consisted of a one sided collapse of the symmetrical position of the abscission zone. The changes in satsuma mandarin were similar to those in ‘Kiyomi’. This implies that the different patterns of morphological changes in the abscission zone cells in ponkan, ‘Kiyomi’, and satsuma mandarin depend on the different locations of their abscission zones. Overall, the results suggest that the cue for fruit abscission in early abscised fruit occurs until 30 h after blocking the carbohydrate translocation to the fruit under 25°C.
In citrus, physiological fruit drop is one of the major problems in commercial fruit production. For primary physiological fruit drop, ovary abscission occurs at the junction between the peduncle and shoot. For secondary physiological fruit drop, fruit abscission occurs at the junction between the fruit and the peduncle (Goren, 1993). Mature fruit abscission also occurs at the junction between the fruit and the peduncle (Goren, 1993). Although many studies have been conducted to elucidate the factors underlying physiological fruit drop, the morphological and anatomical changes in the abscission zone during physiological fruit drop have not been investigated extensively in citrus.
In Arabidopsis, floral organ abscission occurs at the abscission zone, which is characterized as a band of small and dense cytoplasmic cells in the abscising organ (Bleecker and Patterson, 1997). This band of cells, which is formed on a disc across the basal end of the abscission organ connection, is defined as the abscission layer (Gawadi and Avery, 1950). In the tomato, the abscission layer, which is composed of several layers of small cells, expands from the central parenchyma region to the cortical region in the flower pedicel (Tabuchi, 1999), and in the fruit pedicel at a full ripe stage (Tabuchi et al., 2000). This was also observed for the potato flower pedicel (Tabuchi, 1998). In addition, an abscission layer was clearly observed from the epidermal region to the outside of the vascular bundle region at the junction between the calyx and peduncle at anthesis, although it was not observed inside the vascular bundle region in the ovary and young fruit of the Japanese persimmon (Kitajima, 2000). In the mature rabbiteye blueberry, in the region where the pedicel- and peduncle attach, multiple layers of small cells were observed in the vascular tissue, whereas for the region where the pedicel- and fruit are attached, such layer-like cells were absent (Vashisth and Malladi, 2013). However, in mature sour cherry fruit, an abscission layer occurred at the junction between the pedicel and fruit, but not between the pedicel and peduncle (Stosser et al., 1969). Furthermore, in impatiens, leaves abscised without forming an abscission layer at the abscission zone (Gawadi and Avery, 1950). The abscising organ abscises with or without forming an abscission layer, and this can be different among different plant species.
In citrus, an abscission layer was observed between the peduncle and shoot in mature ‘Shamouti’ orange fruit (Huberman et al., 1983) and for the mature ‘Pineapple’ orange between the fruit and peduncle (Wilson and Hendershott, 1967). Furthermore, an abscission layer was formed between the fruit and peduncle after naphthaleneacetic acid (NAA) treatment in the satsuma mandarin during secondary physiological fruit drop (Noma, 1972). However, few histological studies have investigated the formation of abscission zone cells between the fruit and peduncle during secondary physiological fruit drop.
In addition, it is difficult to predict fruit abscission in trees because many variable factors influence physiological fruit drop under field conditions. To investigate the mechanism of fruit abscission, we developed a new method. This consisted of incubating detached fruits in 1% agar medium at 25°C, and abscission induction was developed by blocking the carbohydrate translocation to the citrus fruits (Li et al., 2017). Using this method for several citrus species and cultivars, we characterized the abscission patterns of detached fruits during secondary physiological fruit drop. The cumulative abscission ratio at 96 h after abscission induction in ponkan reached 100%, whereas for hyuganatsu, ‘Kiyomi’, and the satsuma mandarin this was under 50%.
In this study, detached young fruits were incubated in agar medium to initiate fruit abscission induction. The morphological changes in the abscission zone cells were investigated in ponkan (C. reticulata Blanco), hyuganatsu (C. tamurana hort. ex Tanaka), ‘Kiyomi’ (C. unshiu × C. sinensis (L.) Osbeck), and satsuma mandarin (Citrus unshiu Marcow.) during secondary physiological fruit drop.
‘Teishoukei’ ponkan, ‘Futsukei’ hyuganatsu, ‘Kiyomi’, and ‘Miyagawa-wase’ satsuma mandarin, planted at the experimental farm of Kinki University, Wakayama, Japan were used in this experiment. The experiment was performed with 250 open pollinated fruits from leafy inflorescence on each cultivar. All 250 open pollinated fruits were detached from the trees at 4 weeks after anthesis (WAA) in 2012, and they were incubated in 1% agar medium under 25°C for fruit abscission induction. From these fruits, 50 were investigated for their fruit abscission ratio per species and cultivar. The number of abscised fruits was counted every 6 h after abscission induction. The cumulative percentage of fruit abscission was calculated as the cumulative number of abscised fruits divided by the total number of fruits. The other 200 fruits were used as histological samples to investigate the morphological changes in the abscission zone during the fruit abscission process. Up to 10 unabscised fruits were collected every 6 h, 0–72 h after fruit abscission induction. In addition, a maximum of five fruits were collected just after fruit abscission.
Collected fruits were cut from the top of the sepal, including the connecting part between the young fruit and sepal, and fixed in FAA (formalin-glacial acetic acid-70% ethanol, 5-5-9 v/v/v). After fixation, the samples were dehydrated with an ethanol series. After substituting ethanol with Technovit 7100 resin (Heraeus Kulazer, Wehrheim, Germany), the materials were embedded in resin and coagulated in a mold. Transverse sections of 2 μm thickness were prepared with a rotary microtome, stained with toluidine blue O, and observed with a light microscope (BX50; Olympus, Tokyo, Japan). More than eight embedded blocks were prepared at each time for observation. The developmental process of the abscission zone and morphological changes of the cells were observed.
Figure 1 shows the abscission patterns of detached ponkan, hyuganatsu, ‘Kiyomi’, and satsuma mandarin fruit incubated in 1% agar medium under 25°C for 96 h after abscission induction at 4 WAA in 2012. The fruit abscission of ponkan began at 36 h after induction (HAI), with a 4% cumulative abscission ratio. The cumulative abscission ratio subsequently increased and reached 100% at 84 HAI. However, the abscission of ‘Kiyomi’ initiated at 36 HAI, and the cumulative abscission ratio was 22% at 96 HAI. In addition, the abscission of satsuma mandarin initiated at 54 HAI and at 60 HAI for hyuganatsu, and their cumulative abscission ratio at 96 HAI was only 6% and 4%, respectively.

The pattern of detached fruit abscission in ponkan, hyuganatsu, ‘Kiyomi’, and satsuma mandarin, incubated in 1% agar medium under 25°C at 4 weeks after anthesis.
The morphology of abscission zone cells was investigated in ponkan, hyuganatsu, ‘Kiyomi’, and satsuma mandarin at 0 HAI (Fig. 2I–IV), and just after fruit abscission (Fig. 2i–iv). In ponkan, at 0 HAI, the abscission zone consisted of a compact mass of small round cells between the periderm and vascular tissue region, and vertically arranged parenchyma cells within the vascular tissue region. No layer-like cells were observed at the abscission zone (Fig. 2I, Im). For hyuganatsu, ‘Kiyomi’, and satsuma mandarin the morphology of abscission zone cells was similar to that in ponkan at 0 HAI, and layer-like cells were not observed either (Fig. 2II, IIm, III, IIIm, IV, IVm).
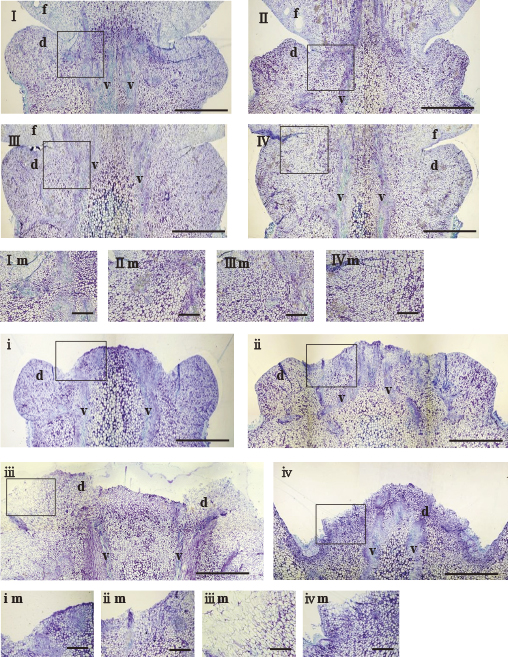
Longitudinal sections of the fruit abscission zone at 4 weeks after anthesis. The abscission zone of a fruit and magnified images of the surrounding part in ponkan (I, Im), hyuganatsu (II, IIm), ‘Kiyomi’ (III, IIIm), and satsuma mandarin (IV, IVm). The distal abscised peduncle side just after fruit abscission in agar medium and magnified images of the surrounding part in ponkan (i, im), hyuganatsu (ii, iim), ‘Kiyomi’ (iii, iiim), and satsuma mandarin (iv, ivm). f: fruit, d: disc, v: vascular bundle. Bars indicate 1.0 mm in fruit images (I, II, III, IV, i, ii, iii, iv) and 200 μm in magnified images (Im, IIm, IIIm, IVm, im, iim, iiim, ivm).
Just after fruit abscission in ponkan and hyuganatsu, fruit abscission occurred at the connected part between the fruit and disc tissue (Fig. 2i, ii). The separated surface of the distal abscised peduncle side was flat in appearance with small round cells, and layer-like cells were not observed on the surface (Fig. 2im, iim), and the entire disc tissue was observed at the distal abscised peduncle side. On the other hand, in ‘Kiyomi’ and satsuma mandarin, fruit abscission occurred at the disc tissue (Fig. 2iii, iv). For ‘Kiyomi’, the surface of the separated region was undefined in appearance, and part of the disc tissue was observed at the distal abscised peduncle side (Fig. 2iii). On the separated surface of the disc tissue, layer-like cells were not observed, whereas elongated cells were observed outside of the vascular tissue (Fig. 2iiim). For satsuma mandarin, cell separation occurred at the basal part of the disc tissue (Fig. 2iv), and only a small part of the disc tissue was observed at the distal abscised peduncle side. The surface of the separated region had an undefined appearance, layer-like cells were not observed at the surface, and elongated cells were observed outside the vascular tissue (Fig. 2ivm), which was similar to ‘Kiyomi’. These results show that the abscission zone is located on the connecting part between the fruit and disc tissue in ponkan and hyuganatsu, and located on the disc tissue in ‘Kiyomi’ and satsuma mandarin. In addition, the abscission layer would not form on the abscission zone.
A morphological change in abscission zone cells was observed at 0, 24, 48, and 60 HAI in ponkan and ‘Kiyomi’ (Fig. 3). For ponkan, the abscission zone, which was located at the connecting part between the fruit and disc tissue, consisted of a compact mass of small round cells between the periderm and vascular tissue region. Furthermore, no morphological changes were observed at 24 HAI (Fig. 3PI, PII, Pi, and Pii), whereas at 48 and 60 HAI, morphological changes in abscission zone cells were clearly observed (Fig. 3PIII, PIV, Piii, and Piv). The dye of the cell walls and cell membranes turned light, the cell membranes separated from the cell walls, and the collapsed cells were loosely arranged between the fruit and disc tissue. In addition, no abscission layer with several layers of small cells was observed during the fruit abscission process. For ‘Kiyomi’, the abscission zone was located at the disc tissue, consisted of round cells between the periderm and vascular tissue region, and no morphological changes were observed at 24 HAI (Fig. 3KI, KII, Ki, and Kii). At 48 HAI, morphological changes in the abscission zone cells were clearly observed. The dye of the cell walls and cell membranes turned light, and cells collapsed (Fig. 3KIII and Kiii). At 60 HAI, the abscission zone cells were severely collapsed (Fig. 3KIV). The dye of cell walls and cell membranes almost seemed to have disappeared, and collapsed cells were enlarged (Fig. 3Kiv). The abscission layer was not observed during the fruit abscission process. These results clearly indicate that there were two types of abscission zone: (1) located between the fruit and disc, which was observed in ponkan and hyuganatsu, and (2) located on the disc tissue as observed in ‘Kiyomi’ and satsuma mandarin. Furthermore, these results indicated that the morphological change in abscission zone cells during the fruit abscission process occurred by 48 h after the induction of fruit abscission. In addition, all observations indicated that no abscission layer was formed before the onset of fruit abscission or during the fruit abscission process.

Longitudinal sections of the fruit abscission zone during fruit abscission process in ponkan and ‘Kiyomi’. The abscission zone of detached incubating fruit in agar medium and magnified cells of the surrounded cells at 0 h, 24 h, 48 h, and 60 h after fruit abscission induction in ponkan (PI, Pi, PII, Pii, PIII, Piii, and PIV, Piv) and ‘Kiyomi’ (KI, Ki, KII, Kii, KIII, Kiii, and KIV, Kiv). f: fruit, d: disc. Bars indicate 200 μm in PI, PII, PIII, PIV, KI, KII, KIII, KIV and 20.0 μm in Pi, Pii, Piii, Piv, Ki, Kii, Kiii, Kiv.
Based on the above results, the morphological changes in the abscission zone cells were investigated at 6 h internals by scoring the levels of cell changes. The morphological changes of the abscission zone cells were divided into five levels (Fig. 4). At level 0 (L0), cells were surrounded by a thick, rigid cell wall, and no morphological changes in abscission zone cells were observed (Fig. 4L0). At level 1 (L1), cell membranes were slightly separated from the cell walls (Fig. 4L1). At level 2 (L2), cell membranes were clearly separated from the cell walls, and loosely arranged cells were observed (Fig. 4L2). At level 3 (L3), the dye of the cell walls turned light, cell walls seemed to be clearly dissolved, and cells appeared to be collapsed (Fig. 4L3). At level 4 (L4), the morphological changes in the cell walls and cells were more severe than at level 3 (Fig. 4L4). The levels of the cells were investigated at five regions on the abscission zone, which was symmetric for positions 1 and 2, and position 3 (Fig. 5). The level score of the two symmetric sides in both position 1 and position 2 were divided into a higher score and lower score, and average values were calculated from both.

Morphological characterization of cells at five levels during the fruit abscission induction process. L0: cell changing level 0, no morphological changes of abscission zone cells; L1: cell changing level 1, cell membrane slightly separated from the cell walls; L2: cell changing level 2, cell membranes clearly separated from the cell walls; L3: cell changing level 3, the dye of the cell walls turned light and cells appear to be collapsed; and L4: cell changing level 4, the morphological changes of cell walls and cells are more severe than at L3. Bars indicate 20.0 μm in L0, L1, L2, L3, L4.

Diagram of the abscission zone between the fruit and peduncle in citrus during secondary physiological fruit drop. Positions 1, 2, and 3 represent the observation sites for the abscission zone. Positions 1 and 2 are symmetrical at longitudinal sections of the fruit abscission zone. The position of the numbered circles represent observations in ‘Kiyomi’ and satsuma mandarin and the dotted circles those in ponkan and hyuganatsu. f: fruit, d: disc, c: calyx, v: vascular bundle, and p: peduncle.
For ponkan, the morphological changes in the abscission zone cells initiated at 30 HAI, and both the higher and lower scores for the cellular changes were 0.8 at position 2 (Fig. 6A). At positions 1 and 2, the higher score for cellular changes both increased to 1.8 at 72 HAI, and the lower score increased to 1.6 and 1.7 at 72 HAI, respectively. At position 3, the cells showed almost no change at 72 HAI. The cells located at the symmetrical side (lower and higher) of position 1 and position 2 changed almost synchronously at each observation time during the fruit abscission process. For hyuganatsu, the changes started at 36 HAI, and the scores for the cellular changes at position 1 and position 2 were both below 1.0 at 72 HAI, indicating that cell changes barely occurred during the first 72 HAI.

The pattern of cellular changes at five positions of the abscission zone during the fruit abscission process in ponkan, hyuganatsu, ‘Kiyomi’, and satsuma mandarin. The higher score and lower score of cellular changes in position 1 ( ,
,  ) and position 2 (
) and position 2 ( ,
,  ), respectively, and position 3 (
), respectively, and position 3 ( ). The levels of cell changing score are shown in Figure 4 and a diagram of the fruit abscission zones and the different positions are shown in Figure 5.
). The levels of cell changing score are shown in Figure 4 and a diagram of the fruit abscission zones and the different positions are shown in Figure 5.
For ‘Kiyomi’, the morphological changes in the abscission zone cells began at 30 HAI, and the higher score was 0.3 at position 2 (Fig. 6C). At positions 1 and 2, both the higher scores of cellular changes increased to 3.5 at 72 HAI, whereas both the lower scores were 0 at 72 HAI. At position 3, the cells showed almost no change. The cells of positions 1 and 2 only intensely collapsed in one of the symmetrical sides at each time during the fruit abscission process. This showed that the pattern of morphological changes in the abscission zone cells is different from that in ponkan. For satsuma mandarin, the morphological change in the abscission zone cells started at 48 HAI at position 3, whereas the score for cellular changes at positions 1 and 2 was 0. At position 2, the higher score was 0.6 at 60 HAI, which increased to 3.0 at 96 HAI. The lower score was 1.0. However, at position 1, the higher score of cellular changes was only 1.0 at 96 HAI, whereas the lower score was 0 (Fig. 6D). The score of cellular changes was almost below 1.5 up to 72 HAI at any of the positions. The scores at positions 1 and 2 were very different between the symmetrical sides during the fruit abscission process, which was a similar pattern to that as observed in ‘Kiyomi’.
Overall, these results indicate that there were two types of morphological change patterns for the abscission cells: (1) the cells changed synchronously at symmetrical sides of positions 1 and 2, as observed in ponkan, and (2) in only one of the symmetrical side of positions 1 and 2 the cells collapsed, as observed in ‘Kiyomi’ and satsuma mandarin. For hyuganatsu, there was no clear pattern because of only the small changes in its cells.
In this study, the morphological changes in the fruit-peduncle abscission zone were investigated by incubating detached fruits of four citrus species and cultivars during secondary physiological fruit drop. It has been reported that citrus fruits abscise at the junction between the fruit and peduncle during secondary physiological fruit drop (Goren, 1993). In our study, just after fruit abscission, an entire tissue disc was observed in ponkan and hyuganatsu, whereas only part of a tissue disc was observed in ‘Kiyomi’ and satsuma mandarin, at the distal abscised peduncle part (Fig. 2i–iv). Because of the appearance of disc tissue after fruit abscission, we consider that citrus fruit can be divided into two types, including (1) the ponkan and hyuganatsu type, in which the abscission zone is located on the connecting part between the fruit and disc and (2) the satsuma mandarin and ‘Kiyomi’ type, in which the abscission zone is located at the disc tissue. This finding is reported for the first time in this article. Furthermore, after fruit abscission, enlarged and swollen collapsed cells were observed in ‘Kiyomi’ and satsuma mandarin (Fig. 3). The enlargements of the abscission zone cells has been observed in the junction between the peduncle and shoot in two-month-old ‘Shamouti’ orange fruits, and between the fruit and peduncle in ethylene-treated 6-8-week-old ‘Shamouti’ orange fruits (Huberman et al., 1988). It was also observed in soybean flower pedicels after ethephon treatment (Oberholster et al., 1991), floral abscised organs in Arabidopsis (Bleecker and Patterson, 1997), and petioles of reproductive olive shoots after ethephon treatment (Kitsaki et al., 1999). Previous studies suggested that an expansion of separation zone cells is the result of tensions that exists across intact walls that are released during separation (Sexton and Roberts, 1982). However, swollen cells were not observed in ponkan and hyuganatsu in this study. This difference may be explained by the different sites of the abscission zones.
It is generally considered that formation of an abscission layer is involved in leaf abscission in poinsettia, cotton, and pepper (Gawadi and Avery, 1950). The formation of an abscission layer was observed in the common tomato flower pedicel (Tabuchi, 1999), which was composed of 6–8 layers of small cells at anthesis. In Japanese persimmon, the abscission layer was composed of about seven layers of small cells, and was observed from the epidermis region to the outside of the vascular bundle region at the junction between the calyx and peduncle at least two weeks before anthesis (Kitajima, 2000). In citrus, about 15 layers were characterized between the petiole and the blade (Jaffe and Goren, 1979). Furthermore, an abscission layer that was composed of 7–8 layers and 3–9 layers of small cells on the outside and inside of the vascular bundle, respectively, were observed at the junction between the fruit and peduncle in mature ‘Hassaku’ fruit (Kurogami and Sogabe, 1953). In addition, Wilson and Hendershott (1967) also observed an abscission layer at the junction between the fruit and peduncle in mature ‘Pineapple’ orange fruit. Furthermore, during secondary physiological fruit drop, an abscission layer is formed between the fruit and peduncle after NAA treatment in young satsuma mandarin fruit (Noma, 1972). In this study, however, no abscission layer was observed at the fruit abscission zone before the onset of fruit abscission, and just after fruit abscission in four citrus species and cultivars during secondary physiological fruit drop (Fig. 2). Morphological changes were observed in abscission zone cells without the formation of abscission layers, and cells were subsequently separated. These results indicate that a different mechanism can regulate fruit abscission during secondary physiological fruit drop in citrus compared to other kinds of fruit with an abscission layer.
In addition, morphological changes in the abscission zone cells were investigated at 0, 24, 48, and 60 HAI during the fruit abscission process in ponkan and ‘Kiyomi’ (Fig. 3), which characterized the morphological changes in the abscission zone cells. Abscission zone cell changes were observed from 48 HAI to 60 HAI in both ponkan and ‘Kiyomi’. For ponkan, the dye of the cell walls and cell membranes turned light, cell membranes separated from cell walls, and collapsed cells were loosely arranged between the fruit and disc tissue. For ‘Kiyomi’, the dye of the cell walls and cell membranes turned light, cells collapsed at 48 HAI (Fig. 3Kiii), cells changed markedly, and collapsed cells were enlarged at 60 HAI (Fig. 3Kiv). The collapsed cells and reduction of the staining in ponkan and ‘Kiyomi’ indicates that cell wall components, such as the middle lamella of cells and pectin, degrade rapidly. This degradation of cell wall components at the abscission zone is related to enzymatic action, such as cellulase and pectin methylesterase. Cellulase and pectin methylesterase induction is associated with organ abscission, and the activity of cellulase and polygacturonase increased during ethylene-induced abscission in the fruit pedicel abscission zone of ‘Valencia’ orange fruits (Jacquelin et al., 1998). Furthermore, a pectin methylesterase gene was preferentially expressed in the laminar abscission zone of citrus leaves (Agustí et al., 2008).
According to observations of cellular changes in ponkan and ‘Kiyomi’, the morphological changes in abscission zone cells are divided into five levels during the fruit abscission process. Based on the level scores, morphological changes in five regions of the abscission zone cells were investigated in ponkan, hyuganatsu, ‘Kiyomi’, and satsuma mandarin. As for the pattern of fruit abscission, the cumulative abscission ratio reached 100% at 96 HAI in ponkan, but only 22% at 78 HAI in ‘Kiyomi’, and below 10% at 96 HAI in both hyuganatsu and satsuma mandarin (Fig. 1). In the morphological investigation, the abscission zone cells for ponkan extended from the epidermis region to the vascular bundle region, and cells changed uniformly in the symmetrical sites of positions 1 and 2 from 30 HAI to 72 HAI (Fig. 6A). However, in ‘Kiyomi’, cells in the symmetrical sides of positions 1 and 2 changed only in one side intensely, and collapsed from 30 HAI to 72 HAI (Fig. 6C). In satsuma mandarin, the average score of cells changing was almost below 1.5 until 72 HAI, and increased to 3.0 at 96 HAI. The morphological changes in the abscission zone cells at positions 1 and 2 collapsed at one of the symmetrical sides (Fig. 6D), which was similar to the changes in ‘Kiyomi’. The high cumulative fruit abscission ratio in ponkan appears to result from the synchronized changes in abscission zone cells, whereas the one-sided collapsing cells of the abscission zone in ‘Kiyomi’ appears to be caused by a low cumulative fruit abscission ratio. The low cumulative fruit abscission ratio of satsuma mandarin seems to have two causes: a low average score of cells changing during the fruit abscission process, and cells changing non-uniformly in the symmetrical sites of positions 1 and 2. In hyuganatsu, changes in abscission zone cells barely occurred from 36 HAI to 72 HAI (Fig. 6B). This resulted in a low cumulative abscission ratio in hyuganatsu. This indicates that the pattern of morphological changes in abscission zone cells during the fruit abscission process in ponkan was different from that in ‘Kiyomi’ and satsuma mandarin. That is, synchronous changes in the abscission zone cells occur in the abscission zone of the connected part between the fruit and disc tissue observed in ponkan. On the other hand, one-sided changes in abscission zone cells in the disc tissue were observed in ‘Kiyomi’ and satsuma mandarin. These different patterns of cell changes seem to be related to the different locations of abscission zones. This suggests that the detailed mechanisms of abscission zone cell separation are different in citrus, depending on the position of the abscission zone.
Initiation of detached fruit abscission happened at 36 HAI in ponkan and ‘Kiyomi’, 54 HAI in hyuganatsu, and 60 HAI in satsuma mandarin (Fig. 1). The morphological changes in abscission zone cells were initiated at 30 HAI in ponkan and ‘Kiyomi’, 36 HAI in hyuganatsu, and 48 HAI in satsuma mandarin (Fig. 6). For citrus, it has been reported that carbohydrate availability for growing young fruits is strongly associated with fruit abscission during physiological fruit drop (Iglesias et al., 2003). In this study, fruit abscission induction was conducted by blocking the carbohydrate translocation to growing young fruits, and the cue for fruit abscission occurred before the morphological changes in abscission zone cells. More specifically, in ponkan, we considered that the cue for fruit abscission would have already occurred around 30 h after blocking of carbohydrate translocation to the fruit under 25°C because genes related to modifying cell walls are expressed before the onset of morphological cell changes. In ponkan, the higher average score of cellular changes slowly increased from 1.5 to 2.0 after 54 HAI, although the cumulative abscission ratio rapidly increased. The maximum score in ponkan was 3, which rarely occurred. Therefore, it is suggested that fruit abscission in ponkan occurs when cells reach a score of 3. In ‘Kiyomi’, on the other hand, the higher average score for cellular changes rapidly increased from around 2.0 to over 3.5 after 60 HAI, while the cumulative fruit abscission ratio hardly changed. In ‘Kiyomi’, the score of 4 appeared regularly during the fruit abscission process. This suggests that fruit abscission in ‘Kiyomi’ may not occur until the lower score for cellular changes reaches 3.
In this study, we propose that the cue for fruit abscission in early abscised ponkan fruit occurs up to 30 h after blocking of carbohydrate translocation to the fruit under 25°C. However, it is important to determine the precise cue time of fruit abscission to study the fruit abscission process. The degradation of cell wall components at the abscission zone is related to enzymatic action, such as cellulase and pectin methylesterase (Agustí et al., 2008; Jacquelin et al., 1998). In addition, hormones play an important role as positive regulators of abscission (Brown, 1997). In citrus, fruit abscission has been considered to follow the concept of an auxin gradient (Okuda, 1999). Auxin moving from the distal to abscission zone tends to delay abscission, and auxin moving down the stem accelerates abscission (Adicott, 1982). On the other hand, the amount of ABA and ACC induced by an earlier carbohydrate shortage increased the onset of primary physiological fruit drop in citrus (Gómez-Cadenas et al., 2000). Recently, microarray analyses were conducted to characterize the differentially expressed genes that regulate the ethylene-induced leaf abscission process in citrus. (Agustí et al., 2008, 2009). Therefore, microarray analyses are necessary to identify the transcriptomic activity of genes related to cell wall degradation and genes encoding for the biosynthesis of hormones during the fruit abscission process in these citrus species and cultivars.
In conclusion, no abscission layers were observed in the fruit abscission zone during the fruit abscission process in four citrus species and cultivars for secondary physiological fruit drop. Fruit abscission occurred in ponkan and hyuganatsu at the part connecting the young fruit and disc tissue, and it occurred at the disc tissue in ‘Kiyomi’ and satsuma mandarin. This indicates that there are at least two different abscission zone sites in citrus species and cultivars. On the other hand, the pattern of morphological changes in the abscission zone cells in ‘Kiyomi’ and satsuma mandarin was different from that in ponkan during the fruit abscission process. This difference appears to relate to the different abscission zone sites. Moreover, it is suggested that under 25°C the cue of fruit abscission in early abscised fruit occurs within 30 h after carbohydrate blocking to the fruit.