2018 Volume 87 Issue 1 Pages 97-105
2018 Volume 87 Issue 1 Pages 97-105
Blossom-end rot (BER) in tomato has been generally reported as a calcium (Ca)-related physiological disorder influenced by cultivar and environmental factors. In our previous works, we found that different fruit-sized cultivars could share a similar threshold value of water-soluble Ca. In addition, seasonal susceptibility to BER was closely related to fruit growth rate. This study aimed to clarify the effect of fruit growth rate as a dominant factor determining the susceptibility in different fruit-sized tomato cultivars. A large-sized cultivar, ‘Momotaro Fight’, and medium-sized ‘Cindy Sweet’, with different susceptibility to BER disorder, were hydroponically grown with modified Hoagland nutrient solutions consisting of a range of Ca:K (potassium) ratios in four cropping seasons. In spring and summer, BER incidence was more than 60 and 10% in ‘Momotaro Fight’ and ‘Cindy Sweet’, respectively, when plants were fed with low Ca. BER was rarely observed when water-soluble Ca exceeded 0.30 μmol·g−1 FW, and the rate of BER incidence increased with a decrease in water-soluble Ca concentration in both cultivars. Fruit growth rate was much more vigorous in ‘Momotaro Fight’ than ‘Cindy Sweet’, especially in summer. It was significantly favored by the increased temperature and solar radiation in both cultivars. The multiple regression analyses detected a significant effect of fruit growth rate on BER incidence, exclusively in ‘Momotaro Fight’. Together with water-soluble Ca, fruit growth rate explained over 50% of the variation of BER incidence. A vigorous rate of fruit growth can play a more important role in decreasing water-soluble Ca in ‘Momotaro Fight’, and result in severe and frequent BER incidence, compared to ‘Cindy Sweet’. Thus the cultivar difference in the susceptibility to BER is likely explained by the difference in the growth rate of young fruit affecting water-soluble Ca in the distal part of tomato fruit.
Blossom-end rot (BER) has generally been reported as a calcium (Ca)-related physiological disorder as there is a well-established relationship between Ca deficiency and incidence of BER in fruit (Adams and Ho, 1993; De Freitas et al., 2014; Lyon et al., 1942; Malavolta et al., 1975; Paiva et al., 1998; Raleigh and Chucka, 1944; Taylor et al., 2004). Recent studies have reported that BER can be triggered by a cellular localized Ca deficiency. Its symptoms start with leaky membranes, cell plasmolysis, and membrane breakdown (De Freitas and Mitcham, 2012; Ho and White, 2005).
The occurrence of BER is influenced by various factors including cultivar and environmental factors (Adams and Ho, 1992; Ho and White, 2005; Ho et al., 1993; Yoshida et al., 2014). It has been reported that BER is enhanced by water stress (Adams and Ho, 1993; Ho and White, 2005; Kataoka et al., 2017; Pill et al., 1978; Robbins, 1937), high temperature, and high light intensity (Adams and Ho, 1993; De Freitas and Mitcham, 2012; Ho, 1989; Ho and White, 2005; Ho et al., 1993; Yoshida et al., 2014). Previous works also showed that the susceptibility to BER disorder differed among cultivars. For example, large fruit-sized cultivars often exhibit frequent BER occurrence while cherry tomatoes are rarely affected (Ho and White, 2005). These environmental or genetic factors may lead to a reduction of Ca uptake into either the root or fruit, or inadequate Ca translocation within the plant or fruit, which finally results in the development of BER. However, the involvement of multiple factors in BER and their interactions are overly complicated. It has not been fully elucidated how each factor leads to Ca deficiency, or to what extent the factor affects Ca deficiency and BER occurrence.
Rapid fruit expansion is believed to be a dominant factor to dilute fruit Ca concentration and increase fruit susceptibility to BER during the fruit enlarging period (De Freitas and Mitcham, 2012; Dekock et al., 1982; Ikeda et al., 2017; Ooyama et al., 2016; Wui and Takano, 1995). Fruit growth is favored under certain conditions of high temperature and solar radiation, perhaps due to accelerated metabolism and increased photoassimilate supply to the fruit (Ho and White, 2005; Ho et al., 1993). Rapid fruit expansion may result in a lag in Ca transport to the distal fruit tissue along with an increase in Ca demand (De Freitas and Mitcham, 2012; Ho and White, 2005; Saure, 2005). However, there is a lack of evidence of clarifying how the fruit growth rate relates to the susceptibility to BER. A comparison between two different fruit-sized cultivars will give us a good opportunity to evaluate the effect of fruit growth on BER development.
However, it is difficult to evaluate the effect properly if cultivars have different responses to critical Ca level in developing BER symptoms. Unlike most works that concern total Ca within fruit tissues (Ho and White, 2005), we found that water-soluble Ca representing apoplastic Ca, cytoplasmic Ca, and loosely wall-bound Ca in the distal part of tomato fruit was potentially most related to the physiological process of BER development in a large sized tomato cultivar, ‘House Momotaro’ (Yoshida et al., 2014). In a large-sized cultivar, the frequency of BER development increased when water-soluble Ca decreased below 0.20 μmol·g−1 FW in the distal part of the young fruit. In a further study on a medium-sized cultivar ‘Cindy Sweet’, we demonstrated that it showed similar threshold value of water-soluble Ca to the large-sized cultivar, even though it is less susceptible to BER (Ooyama et al., 2016). Therefore, a comparative analysis of different fruit-sized cultivars, which showed similar critical values of Ca, will provide accurate information on the development of BER.
In the present study, two different fruit-sized cultivars, ‘Momotaro Fight’ which is similar to ‘House Momotaro’ and ‘Cindy Sweet’, characterized with different susceptibility to BER disorder were examined to clarify the effect of fruit growth rate on the susceptibility to BER. In addition, we attempted to investigate the seasonal changes of fruit growth and water-soluble Ca level, and their relation to BER incidence by four croppings.
Four experiments were carried out in a plastic house (6 m wide × 19 m long × 4 m high) in the Faculty of Agriculture, Okayama University from February 2013 to June 2014, which included spring (February–June), summer (April–July), and autumn (September–November) of 2013 and spring (March–June) 2014. To control growing conditions, the temperature in the plastic house was maintained above 10°C by a warm-air heater and adequate ventilation was applied with fans and windows when the temperature exceeded 28°C.
Values of environmental factors, including temperature, solar radiation, and day length, were averaged during the interval of treatment (from the anthesis of the first flower to the end of fruit sampling) in each experiment (Table 1). Mean temperatures were 24.2, 29.7, 21.4, and 22.2°C in spring, summer, and autumn 2013 and spring 2014, respectively. On average, solar radiation was only lower in autumn 2013 compared to the other seasons.

Growing conditions and flowering of ‘Momotaro Fight’ and ‘Cindy Sweet’ in four experiments (February 2013 to June 2014).
Two tomato (Solanum lycopersicum L.) cultivars including a large round fruiting cultivar ‘Momotaro Fight’ (Takii, Kyoto, Japan) and a medium-sized fruiting cultivar ‘Cindy Sweet’ (Sakata Seed, Yokohama, Japan), that are characterized with different susceptibility to BER disorder, were examined. The seeds were sown on vermiculite moistened with water and then transplanted to rock wool cubes (75 mm × 75 mm × 65 mm; Nitto Boseki Co. Ltd., Tokyo, Japan) when the third true leaf had emerged. Seedlings were fertigated daily with modified Hoagland solution (4/4 of Ca/potassium (K) in Table 2; Hoagland and Arnon, 1950) after germination. Treatments were applied to plants when their first flowers were visible. Tomato plants were hydroponically nourished on NFT troughs (one trough = one treatment, 1.8 m long and 0.3 m wide) with modified nutrient solutions containing 1/7 to 5/3 (me·L−1) of Ca/K by replacing Ca nitrate [Ca(NO3)2] and K nitrate (KNO3) appropriately (Table 2). Solutions with ratios ranging from 2/6 to 5/3 were applied in the experiments during spring 2013, summer 2013, and spring 2014 and Ca/K ratios of 1/7 to 4/4 were applied in autumn 2013 in order to develop the variation in BER incidence as affected by different levels of Ca nutrition (Table 1). Tap water containing 0.2 mmol·L−1 Ca and 0.1 mmol·L−1 magnesium (Mg) with an EC value of 8–10 mS·m−1 was used for the preparation of the nutrient solution. The EC of the nutrient solution was in the range of 120–130 mS·m−1.

Nutrient composition used for experimental treatments (mmol·L−1).
Ion absorption (A, mmol) was determined via measurement of the rate of depletion in the solution as shown in the following equation:
On each NFT trough, four plants of ‘Momotaro Fight’ and eight of ‘Cindy Sweet’, with no border plants, were placed 15 cm apart and trained alternately into two rows 50 cm apart. Each nutrient solution was circularly supplied from a small tank containing 6 L of nutrient solution (0.5 L per plant), which was automatically filled with the nutrient solution from a large tank via a float switch. The nutrient solution was circulated for 15 min per h during the day (06:00–18:00) and 15 min per 3 h at night (18:00–06:00) by a time switch. The sympodial shoot of each plant was pinched, leaving two leaves above the third truss.
Date of anthesis was recorded for all flowers on each truss of 12 plants in each treatment. A 15 mg·L−1 solution of 2-methyl-4-chlorophenoxyacetic acid (4-CPA) was sprayed to guarantee uniform fruit set period. Dates of visible BER symptoms were recorded, and the incidence of BER calculated as a percentage by dividing the number of BER-affected fruits by the total number of fruit on each truss of tomato plants in each treatment.
The second fruit of each truss was sampled for Ca extraction at 14 and 18 days after anthesis for ‘Momotaro Fight’ and ‘Cindy Sweet’ in the four experiments, respectively. Rate of fruit growth was calculated by dividing fruit weight by the number of days after anthesis. Ca in the distal part of the fruit was sequentially extracted to water-, sodium chloride (NaCl)-, and hydrochloric acid (HCl)-soluble Ca fractions that served as representatives for (1) apoplastic and cytoplasmic Ca2+, loosely wall-bound Ca; (2) Ca pectate in cell wall; and (3) insoluble Ca phosphate and Ca oxalate, respectively. The procedure for fractionated Ca extractions was done as described in Yoshida et al. (2014). Ca concentration was determined using atomic absorption spectrometry (SPCA-6210; Shimadzu, Kyoto, Japan) and described as μmol·g−1 FW.
To predict the response of BER incidence to water-soluble Ca in the distal part of the fruit, a logistic sigmoid function curve is estimated with the following equation: y = 100/(1 + ea(x − b)) where y is the incidence of BER, x is the water-soluble Ca concentration, a is the gain coefficient, and b is the value of x at the midpoint (Kent et al., 1972; McDowall and Dampney, 2006). Microsoft Excel spreadsheets and the program R were used for data analysis.
Plants of the two cultivars were grown under the same rhizosphere conditions to allow determination of the difference in susceptibility of fruits between cultivars. Similar to our previous report (Yoshida et al., 2014), increased Ca concentration in the nutrient solution significantly increased Ca absorption but decreased K absorption (Table 3). The varied Ca/K ratios in the nutrient solutions did not affect plant absorption of water and other major elements (data not shown). Among growing seasons, the differences in the absorption of Ca and other elements were small except for autumn 2013.

Effect of Ca concentration (me·L−1) in the supplied solutions on apparent Ca and K absorption (mmol/plant·day−1) of tomato plants in the four experiments (February 2013 to June 2014). Values are means of each plot during the experiments.
There was no difference in vegetative growth of tomato plants among the plots supplied with different solutions. Seasonally, the least vigorous growth was in autumn 2013 and the other experiments showed only small differences. The varied Ca concentrations in the nutrient solution did not significantly affect flowering and fruit growth rate within each cultivar (data not shown).
Fruit growth rate was higher in ‘Momotaro Fight’ than in ‘Cindy Sweet’ throughout the experiments (Fig. 1). The difference was most remarkable in summer 2013 when the fruit growth rate of ‘Momotaro Fight’ was approximately twice that of ‘Cindy Sweet’. Among seasons, the rate of fruit growth was in the order: summer 2013 > spring 2014 > spring 2013 > autumn 2013 in both cultivars, except in spring and autumn 2013 for ‘Cindy Sweet’.
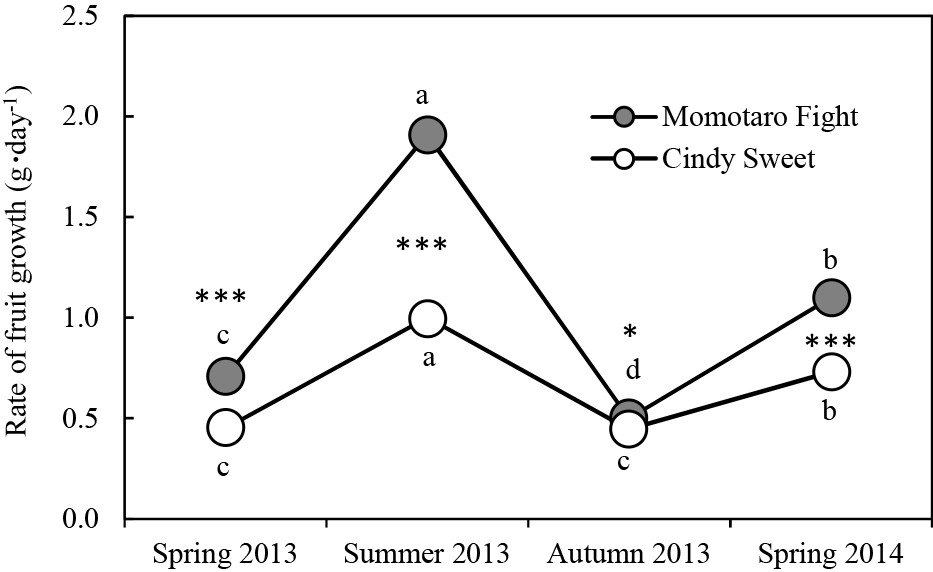
Changes in growth rate (g·day−1) of young fruits of ‘Momotaro Fight’ and ‘Cindy Sweet’ at 14 and 18 days after anthesis, respectively. Values with different letters in each cultivar are significantly different among seasons (n = 12). * and *** indicate significant difference at P < 0.05 and P < 0.001 between two cultivars in each season, respectively.
The effects of environmental factors on fruit growth rate are shown in Table 4. In both cultivars, fruit growth rate was strongly affected by temperature and solar radiation. Over 55 and 40% of the variation in fruit growth rate was determined by temperature and solar radiation for ‘Momotaro Fight’ and ‘Cindy Sweet’, respectively, when the values of individual fruits were pooled (n = 191 and 377) in a multiple regression against temperature and solar radiation. Fruit growth of ‘Momotaro Fight’ was more favored by temperature compared to ‘Cindy Sweet’, as the slope values for temperature and solar radiation in ‘Momotaro Fight’ were approximately 2.4 and 1.4 times the values for ‘Cindy Sweet’, respectively.
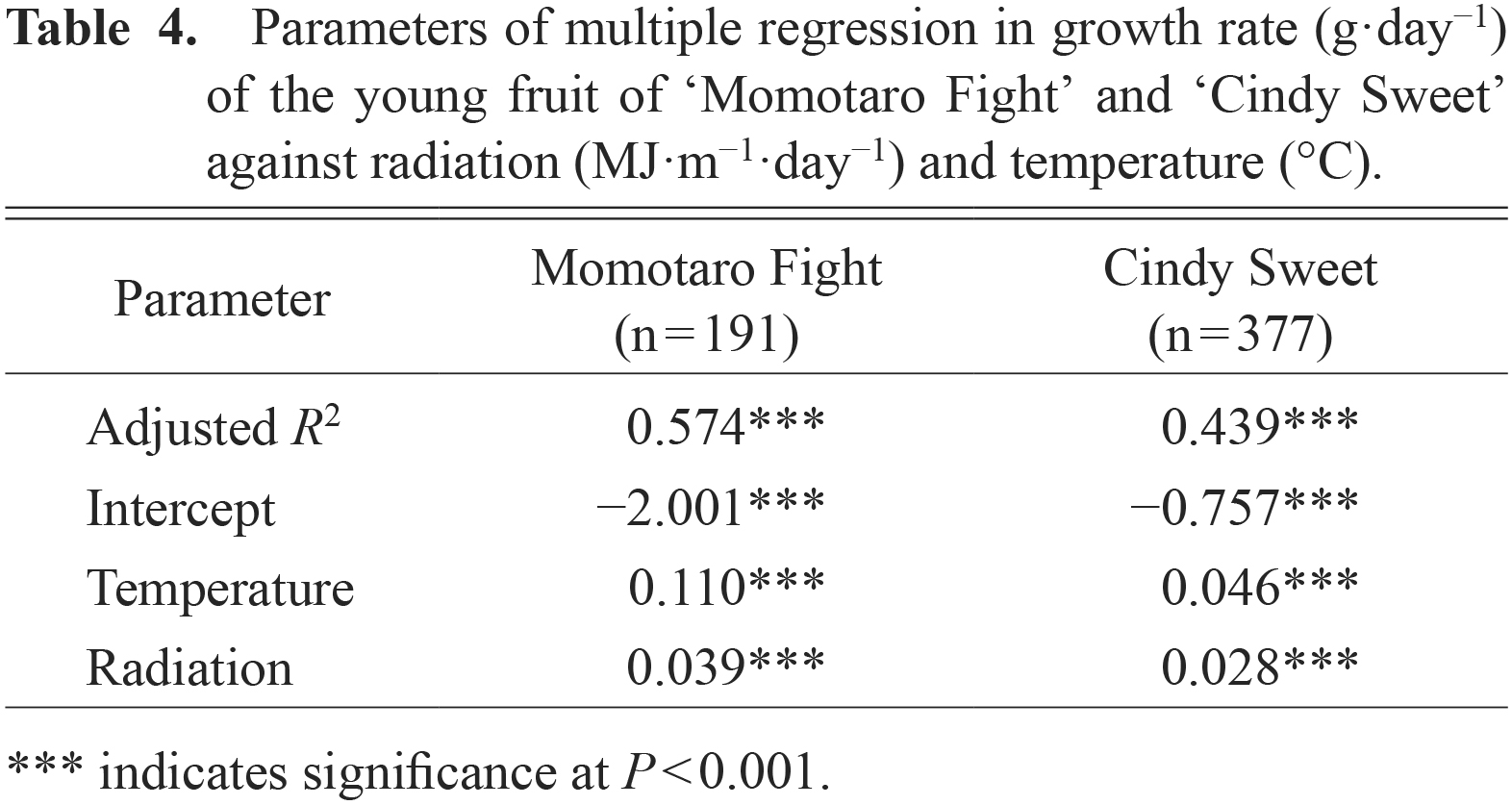
Parameters of multiple regression in growth rate (g·day−1) of the young fruit of ‘Momotaro Fight’ and ‘Cindy Sweet’ against radiation (MJ·m−1·day−1) and temperature (°C).
The BER incidence increased linearly with decreasing Ca/K ratios in the supplied solution and remarkably differed between the two cultivars despite being hydroponically nourished under the same environmental conditions (Fig. 2). ‘Momotaro Fight’ had a high rate of BER incidence while ‘Cindy Sweet’ was hardly affected by BER even at low Ca concentration in the supplied solution. In ‘Momotaro Fight’, the BER incidence was much higher in summer 2013 and spring 2014 than in spring and autumn 2013. The symptoms of BER appeared and developed most quickly in summer 2013, followed by spring 2014, spring 2013, and lastly by autumn 2013 (data not shown). In contrast, the variation in BER incidence among seasons was small for ‘Cindy Sweet’ compared to ‘Momotaro Fight’.

The averaged percentage of BER incidence in ‘Momotaro Fight’ and ‘Cindy Sweet’ as affected by Ca concentration in the supplied solutions and growing season. ** indicates significance at P < 0.01.
The mean values of total and water-soluble Ca concentrations in the distal part of the young fruit and the results of three-way analysis of variance (ANOVA) for three common treatments with Ca in solutions of 2, 3, and 4 (me·L−1) throughout the four experiments are shown in Table 5. Correspondingly, total and water-soluble Ca concentrations were higher in ‘Cindy Sweet’ than in ‘Momotaro Fight’. Increasing Ca concentration in the supplied solution resulted in increased total and water-soluble Ca concentrations in both cultivars. However, there were no significant differences in total and water-soluble Ca between fruits grown with Ca in solutions of 4 and 5 (me·L−1) for the three experiments, except for autumn 2013 (data not shown). Seasonal differences were significant for total and water-soluble Ca. The difference in water-soluble Ca concentration was smaller in ‘Cindy Sweet’ compared to ‘Momotaro Fight’, consistent with the small variation in BER incidence among seasons.

Changes in total and water-soluble Ca concentrations (μmol·g−1 FW) in the distal part of the young fruit of ‘Momotaro Fight’ and ‘Cindy Sweet’.
The relationships between water-soluble Ca concentration in the distal part of the young fruit from each truss and the percentage of BER-affected fruits in both cultivars were plotted in Figure 3. Similar to our previous reports (Ooyama et al., 2016; Yoshida et al., 2014), significant negative linear relationships were observed for both ‘Momotaro Fight’ (R2 = 0.499***) and ‘Cindy Sweet’ (R2 = 0.261***). BER was most apparent on the truss in which the distal part contained <0.18 μmol·g−1 FW of water-soluble Ca. BER occurred occasionally when the concentration was in the ranges of 0.18–0.30 μmol·g−1 FW. The fruit was hardly affected by the BER when water-soluble Ca exceeded 0.30 μmol·g−1 FW.

Relationships between BER incidence (%) and mean value of water-soluble Ca concentration (μmol·g−1 FW) in the distal part of the young fruit in each truss.
In ‘Momotaro Fight’, the logistic sigmoid curve (y = 100/(1 + e30.59(x − 0.15)); R2 = 0.602) represents a BER incidence rate of 50% when water-soluble Ca in the distal part decreases to 0.15 μmol·g−1 FW; 20% at water soluble Ca of 0.20 μmol·g−1 FW, and 1% when water-soluble Ca reaches 0.30 μmol·g−1 FW. As less susceptible to BER, ‘Cindy Sweet’ represents 7, 4, and 1% BER incidence when water-soluble Ca decreases to 0.15, 0.20, and 0.30 μmol·g−1 FW, respectively.
Factors affecting water-soluble Ca concentration in the distal part of fruit and BER incidenceThe effects of radiation and temperature, together with Ca in nutrient solution and order of truss, were pooled into a multiple regression to examine their effects on water-soluble Ca in the distal part of the young fruit (Table 6). Similar to our data previously described for the cultivar ‘House Momotaro’ (Yoshida et al., 2014), Ca in nutrient solution indicated a promotive effect on water-soluble Ca in both cultivars. The increase in the order of truss and temperature also had negative effects on water-soluble Ca in both cultivars. Remarkably, water-soluble Ca in ‘Momotaro Fight’ was more sensitive to temperature variation compared to ‘Cindy Sweet’, as the slope value of temperature for ‘Momotaro Fight’ was approximately 1.7 times that for ‘Cindy Sweet’. However, the effect of solar radiation was very small in both cultivars.

Parameters of linear multiple regression in water-soluble Ca concentration (μmol·g−1 FW) in the distal part of fruit against Ca in solution (me·L−1), the order of truss, daily radiation (MJ·m−2·day−1), and temperature (°C).
Table 7 shows the effects of Ca absorption and rate of fruit growth on water-soluble Ca concentration in the distal part of the young fruit. Water-soluble Ca was significantly increased by the increase in apparent Ca absorption. In ‘Momotaro Fight’, fruit growth rate significantly diluted water-soluble Ca in the distal part of the fruit. However, the effect of fruit growth rate was very small for ‘Cindy Sweet’. In addition, fruit growth rate also aggravated the susceptibility of ‘Momotaro Fight’ to BER but did not significantly affect ‘Cindy Sweet’ (P = 0.61) (Table 8). In ‘Momotaro Fight’, over 52% of the variation in BER incidence was explained by water-soluble Ca concentration in distal fruit tissue and fruit growth rate. The coefficient of determination in ‘Cindy Sweet’ was only approximately 24%, indicating a small effect of fruit growth rate.

Parameters of linear multiple regression in water-soluble Ca concentration (μmol·g−1 FW) in the distal part of fruit against apparent Ca absorption (mmol/plant·day−1) and rate of fruit growth (g·day−1).

Parameters of linear multiple regression in the rate of BER incidence against mean values of water-soluble Ca concentration in the distal part of fruit (μmol·g−1 FW) and rate of fruit growth (g·day−1) in each truss.
BER is often observed during the phase of rapid fruit growth 2–3 weeks after anthesis (Adams and El-Gizawy, 1988; Ho and White, 2005; Saure, 2001; Sonneveld and Voogt, 1991; Wui and Takano, 1995). In this study, we examined two cultivars with different sizes and susceptibility to BER under hydroponic condition. In the susceptible cultivar ‘Momotaro Fight’, the logistic sigmoid fitting model reveals that 50 and 20% of BER incidence are estimated when water-soluble Ca concentration in the distal part of the young fruit decreases to 0.15 and 0.20 μmol·g−1 FW, respectively. The corresponding values for ‘Cindy Sweet’ are 7 and 4% at the same levels of water-soluble Ca. When water-soluble Ca increases to 0.30 μmol·g−1 FW, BER is rarely triggered in both cultivars (Fig. 3). In our previous works, the possibly critical water-soluble Ca level was also observed from 0.15 to 0.20 μmol·g−1 FW for ‘House Momotaro’ (Yoshida et al., 2014) and ‘Cindy Sweet’ (Ooyama et al., 2016). In the present study, one or more BER fruits were also observed on the trusses where water-soluble Ca decreased below 0.18 μmol·g−1 FW in both cultivars even though the root condition affecting plant water relations was quite different from the previous study. Some authors (De Freitas and Mitcham, 2012; Picchioni et al., 1996) also stated that water-soluble Ca must be maintained at a certain threshold for the stabilization of proper cell structure. It is therefore suggested that an instant and irreversible lack of available Ca2+ in the cell likely induces BER and the measurement of water-soluble Ca concentration in the distal part of the young fruit would be a useful diagnostic approach and the value of 0.20 μmol·g−1 FW may indicate the risk of BER development.
The effects of decreased Ca concentration in nutrient solution on water-soluble Ca (Tables 5, 6, and 7) could be a result of decreased Ca absorption (Yoshida et al., 2014). Actually, root Ca2+ absorption might act as a source loading Ca2+ into vascular bundles, but not directly defining water-soluble Ca in the young fruit. A slightly higher water-soluble Ca concentration and lower BER incidence were observed in autumn 2013, although plants absorbed the least Ca2+ from the nutrient solution compared to other seasons. Low BER incidence was partly due to the lowest rate of fruit growth (Figs. 1 and 2), but the observed high water-soluble Ca (Table 5) might be affected by Ca distribution within a plant.
Temperature and solar radiation are widely known as potential BER-inductive factors. The promotive effects of irradiance and ambient temperature on fruit growth and/or perturbation in Ca uptake and distribution within the whole plant may trigger BER development (Adams and Ho, 1993; De Freitas et al., 2012; Ho and White, 2005). However, no significant effect of radiation was observed against water-soluble Ca concentration in both cultivars (Table 6). Small variation among the three experiments, except autumn 2013, may have affected the results of the regression analysis. Low solar radiation (Yoshida et al., 2014) and also short photoperiod (Ooyama et al., 2017) may reduce water and also Ca competition against leaves caused by transpiration.
The higher susceptibility of ‘Momotaro Fight’ compared to ‘Cindy Sweet’ possibly resulted from the lower water-soluble Ca concentration (Table 5) caused by the higher rate of fruit growth (Tables 7 and 8). It has been reported that the difference in the susceptibility to BER disorder among cultivars in tomato (Adams and Ho, 1992; Ho and White, 2005) and pepper (Marcelis and Ho, 1999) could relate to genetic characteristics regulating the potential size and growth rate of fruit. The requirements of Ca for fruit expansion are widely reported to increase under conditions favoring vigorous fruit growth (Adams and Ho, 1992; Ho and White, 2005; Ho et al., 1993; Saure, 2001, 2005). Therefore, low water-soluble Ca in the susceptible cultivar, characterized by highly vigorous fruit growth, possibly results from a lag in Ca transport to the distal part of the young fruit. Saure (2005) also stated that high growth rate of fruit increased the risk of decreasing Ca below a critical level required for cell stabilization during the enlarging period.
The decrease in water-soluble Ca caused by environmental factors should be a consequence of their promotive effects on fruit growth. Previous works have also shown that fruit growth might be promoted by increased daily solar radiation (Kitano et al., 1998; Sonneveld and Voogt, 1991), increased temperature, or a combination of warm weather and high solar radiation (Ho et al., 1993; Wui and Takano, 1995) due to increased photosynthetic rates (Ho et al., 1993) and consequent photoassimilate translocation into fruit (De Freitas and Mitcham, 2012; Ho and White, 2005; Walker and Ho, 1977). Since the variation in water-soluble Ca in the distal part of the young fruit was highly related to the rate of fruit growth in the susceptible cultivar ‘Momotaro Fight’ (Table 7), it is highly possible that the promotive effects of temperature and solar radiation favoring rate of fruit growth (Table 4) are more significant on cultivars with high fruit growth rate and large potential fruit size, aggravating the susceptibility of these cultivars to BER, compared to small-sized cultivars.
In conclusion, we demonstrated that the cultivar difference in the susceptibility to BER is likely explained by the difference in the growth rate of young fruit, which may closely relate to potential fruit size and majorly defines water-soluble Ca in the distal part of tomato fruit. A vigorous rate of fruit growth in large-sized cultivar, such as ‘Momotaro Fight’, could work as a dominant factor to decrease water-soluble Ca. Under conditions favoring high fruit growth, such as high temperature and strong irradiation, water-soluble Ca can easily decrease below the critical level and a breakdown of Ca homeostasis likely triggers BER development in the young fruit. When the water-soluble Ca, including apoplastic and cytoplasmic Ca2+, in the distal part of the young fruit is higher than 0.30 μmol·g−1 FW, BER symptoms rarely developed in both cultivars. Water-soluble Ca can be a useful risk diagnosing parameter of BER incidence, and the level of 0.20 μmol·g−1 FW may be critical for the frequent development of BER in different sized tomato cultivars grown under various environmental conditions including the rhizosphere. Further investigations should focus on Ca transport, partitioning, and consumption of Ca2+ within fruit during the phase of rapid fruit development to clarify the importance and the threshold value of Ca in the physiological process.