2018 Volume 87 Issue 2 Pages 206-213
2018 Volume 87 Issue 2 Pages 206-213
Several Japanese tomato cultivars develop a physiological disorder called leaf marginal necrosis, which occurs in relatively young compound leaves. Although the positions of the observed symptoms differ from those caused by inadequate potassium (K+) supplementation, previous studies have reported a relationship between the reduction of K+ content and the occurrence of this disorder. However, the mechanism of the relationship between K+ deficiency and leaf marginal necrosis remains unstudied. In the present study, the relationship between K+ deficiency in leaflets and leaf marginal necrosis was investigated by cation measurement and gene expression analysis to understand the possible mechanism responsible for the induction of leaf marginal necrosis. First, cation measurement of the two cultivars differing in their symptom intensities showed a trend of K+ reduction in the ‘CF Momotaro J’ cultivar developing leaf marginal necrosis at the tip leaflets positioned under the flowering fruit truss. Next, a comparison between the basal and tip region of the leaflet from four cultivars differing in their symptom intensities revealed that the K+ concentration in tip leaflets was significantly lower in the tip regions compared to the basal region, especially in the two cultivars ‘CF Momotaro J’ and ‘Momotaro grande’, leading to leaf marginal necrosis. The gene expression analysis of the basal and tip regions identified that the expression patterns of jasmonate-related genes were upregulated in the tomato leaflets with low K+ concentration. The gene expression of a leaf senescence marker gene, a homologue of the SAG12 gene of Arabidopsis thaliana, was detected only in the leaf tip region samples with the lowest K+ concentration. Furthermore, ‘CF Momotaro J’ plants cultivated with K+-supplemented medium showed an increase in the K+ concentration, a decrease in the occurrence of leaf marginal necrosis, and down-regulation of the expression of jasmonate-related genes in tip leaflets. These results indicate that tomato leaf marginal necrosis occurs because of K+ starvation in the tip region of leaflets, leading to the activation of jasmonate-induced signal for necrosis.
The tomato (Solanum lycopersicum) is one of the most important vegetable crops grown worldwide (Tomato Genome Consortium, 2012). Some tomato cultivars develop a physiological disorder called leaf marginal necrosis (Fig. S1). The occurrence of this disease is largely dependent on the tomato cultivars. Its symptoms start with the yellowing (chlorosis) of leaf margins in the tip leaflet, which eventually develops into necrosis of leaf marginal cells. When the symptoms get severe, chlorosis is observed in a relatively larger area of the compound leaf. Although cell death decreases the active leaf area and thus reduces photosynthesis, the greatest damage is caused by infection with Botrytis cinerea in the necrotic regions. This fungus first propagates on the necrotic leaf area and then infects the living leaves, stems, and fruits, thus greatly reducing the number of harvestable fruits.
Previously, Oka et al. (2002) reported that tomato leaves with leaf marginal necrosis symptoms contain a relatively low amount of potassium (K+), but moderate amounts of nitrogen, calcium, magnesium, and phosphate. Additionally, they reported that supplementation of K+ fertilizer in the soil reduced the symptoms of this physiological disorder. Furthermore, Kumazaki et al. (2010) reported that spraying K2SO4 solution on tomato leaves effectively reduced the symptoms of leaf marginal necrosis (which was called burned leaf tip in their report). These two reports indicate an involvement of K+ insufficiency in the occurrence of leaf marginal necrosis. Although these studies have indicated a relation between K+ and leaf marginal necrosis, the causal association between K+ concentration and leaf marginal necrosis remains unknown.
K+ is a cation essential for almost all organisms and plays an important role in fundamental physiological processes such as enzyme activation, osmoregulation, protein synthesis, electrical neutralization, photosynthesis, and control of turgor pressure, in plants (Adams and Shin, 2014; Ahmad and Maathuis, 2014; Ashley et al., 2006; Romheld and Kirkby, 2010; Wang and Wu, 2010). Because of its importance, K+ has been extensively studied to identify the mechanism of its uptake, homeostasis, involvement in plant metabolism and gene expression, and plant reaction to K+ deficiency (Armengaud et al., 2004; Cao et al., 2006; Jung et al., 2009; Ruan et al., 2015; Shahzad et al., 2016; Song et al., 2015; Troufflard et al., 2010; Zeng et al., 2014).
Tomato plants grown in a greenhouse require very large amounts of K+ (Besford and Maw, 1975). In the tomato, an inadequate supply of K+ ions induce chlorosis in the leaf margins, usually beginning in the mature leaves (Mulholland et al., 2001; Pujos and Morard, 1997), unlike leaf marginal necrosis symptoms, which are mainly observed in the leaflets of relatively young compound leaves. Thus, the symptoms of leaf marginal necrosis are distinct from those of plants grown in low K+ conditions.
In this paper, we tried to identify the mechanism of the relationship between K+ and the occurrence of leaf marginal necrosis using commercial tomato cultivars differing in the degree of this physiological disorder, in order to help explore the cultivation conditions that would reduce the symptoms of this disorder in tomato plants. In Experiment 1, the relation between K+ and leaf marginal necrosis was investigated by measuring the K+ concentration in the leaves of two cultivars. Subsequently, in Experiment 2, the mechanism of the induction of leaf marginal necrosis by K+ starvation was studied by a combination of regional cation measurement and gene expression analysis of the stress-related genes, comparing between the basal and tip region of the tomato leaflet. The proposed mechanism of the induction of this physiological disorder by K+ starvation was further confirmed in Experiment 3 through tomato cultivation using a growth medium supplemented with additional K+.
Tomato plants were grown under greenhouse conditions at the Gifu Prefectural Agricultural Technology Center (Gifu, Japan). Two cultivars ‘CF Momotaro J’ (Takii & Co., Ltd., Kyoto, Japan) and ‘Reiyo’ (Sakata Seed Co., Ltd., Kanagawa, Japan) were used for this study (hereafter notated as CMJ and RYO, respectively). The seeds were sown in a cell tray filled with nursery soil on July 24, 2014. The trays were placed in a greenhouse, and the seedlings were transplanted to the soil-containing pots (15-cm pot size, 1.2 L of soil per plant, 20-cm interval between each individual) on August 7. The soil used for this study was based on peat moss, coco peat, vermiculite, and zeolite, and contained no fertilizer. The plants were supplied with “Yamazaki shohou” nutrient solution, which consisted of 6.57 mM NO3−, 3.65 mM K+, 1.45 mM Ca2+, 0.91 mM Mg2+, 0.91 mM SO42−, 0.64 mM NH4+, 0.61 mM PO43−, 0.02 mM Fe2+, 17.8 μM BO33+, 7.5 μM MnO42+, 3.5 μM Zn2+, 0.95 μM Na+, 0.7 μM Cu2+, and 0.5 μM MoO42+ (with an electrical conductivity (EC) of 1 dS·m−1). The amount of nutrient solution supplied to the plants was adjusted from 4 to 40 times a day, based on the plant growth and weather conditions. A system detecting the drain fluid controlled the amount of each supplementation, totally applying 300 to 2000 mL of nutrient solution to each plant per day. The nutrient density was adjusted in terms of EC from 0.5 to 1.4 dS·m−1 by simply diluting or enriching the total nutrient concentration. During plant growth, EC was first adjusted to 0.5 dS·m−1 just after transplantation to pots and then ramped up to 1.0 dS·m−1 at the time of the 1st fruit truss anthesis (FTA), approximately one month after the transplant, based on plant growth. Subsequently, EC was raised to 1.4 dS·m−1 in increments of 0.1 dS·m−1 each at the 2nd, 3rd, 4th, and 5th FTA. The fruit number in each fruit truss was adjusted to four, and the plants were grown until the 6th FTA. At the time of the 3rd, 4th, 5th, and 6th FTA, plant data and leaf samples were collected based on the flowering fruit truss, tip leaflets from the upper and lower compound leaves, and base leaflets from the lower compound leaves (n = 3). The collected tissues were used for cation measurements. The FTA was utilized because it was a suitable index to represent plant growth, and it was thought to be related to the occurrence of leaf marginal necrosis. The average day temperature, average day humidity, and sunshine duration of the cultivation period at Gifu, announced by the Japan Meteorological Agency (http://www.jma.go.jp/jma/index.html), are presented in Figure S2.
Experiment 2: Comparison between the basal and tip region of a tip leafletFour cultivars, including ‘Momotaro grande’ (Takii & Co., Ltd.), ‘Rinka409’ (Sakata Seed Co., Ltd.) (hereafter notated as MoG and RNK, respectively), CMJ, and RYO, were grown in a greenhouse at the Institute of Vegetable and Floriculture Science, NARO (Tsu, Japan). Seeds were lined up on a wet filter paper placed in a petri dish on September 23, 2016, and the germinated seeds were sown in rock wool blocks (6.5 cm × 6.5 cm × 7.5 cm) on September 27. The plants were grown using the same nutrient solution as that in Experiment 1. The nutrient solutions were supplied 4 to 6 times a day, each time applying a sufficient amount to reach the absorption limit of the rock wool. In the beginning, EC was adjusted to 0.2 dS·m−1 and was increased by 0.1 dS·m−1 each in a stepwise manner every 7 to 10 days until it reached an EC value of 1.0 dS·m−1. The basal and tip regions of the tip leaflet from the compound leaves just under the flowering fruit truss were collected at the 3rd FTA (n = 3~4, except for RYO at the 3rd FTA, which had only two replicates) and were used for cation measurement and gene expression analysis. The average day temperature, average day humidity, and sunshine duration of the cultivation period at Tsu, announced by the Japan Meteorological Agency are shown in Figure S3.
Experiment 3: Reaction under additional K+ supplemented medium and the occurrence of leaf marginal necrosisIn this experiment, the same cultivars as those in Experiment 1 were used and grown under the same conditions as those in Experiment 1. The seeds were sown in cell trays on July 22, 2015. The plants were transplanted to pots on August 7 and were grown till the 9th FTA. The fruit number on each fruit truss was adjusted to four, and the harvest of fruits commenced at around the 5th FTA. Three nutrient solutions were used for CMJ, which differed mainly in the concentration of K+ (4 me·L−1, 6 me·L−1, and 8 me·L−1, hereafter notated as control, 6K, and 8K respectively), by supplementing KNO3, K2SO4, and KH2PO4, and by decreasing the amount of NH4NO3 to adjust the concentration of nitrogen (Table S1). The nutrient solutions were applied to tomato plants as described in Experiment 1 for the control plants. For 6K and 8K nutrients, the EC value was set to 0.2 and 0.4 dS·m−1 higher than the control plants, respectively, rendering the concentration of the nutrient equal to that of the control solution, except for K+. The leaf tissue from the tip leaflet was collected at the 4th FTA (n = 6); this was thought to be just before the onset peak of leaf marginal necrosis so as to avoid the contamination of any dead tissues. The average day temperature, average day humidity, and sunshine duration during the cultivation period at Gifu, announced by the Japan Meteorological Agency, are shown in Figure S4.
Measurement of leaf marginal necrosis intensityThe intensity of symptoms was determined as an average of the symptom intensity observed in each individual; it was calculated using the average symptom within five compound leaves located just under the fruit truss at the anthesis stage. The intensity of symptoms in each compound leaf was recorded on a scale of 0 to 4 (0: no symptoms, 1: symptoms observed only in the tip leaflets, 2: symptoms observed in one-third of the leaflets, 3: symptoms observed in two-thirds of the leaflets, and 4: symptoms observed in more than two-thirds of the total leaflets). A photo showing an example of the intensity of symptoms is shown in Figure S5.
Cation measurementsThe plant samples collected in each experiment were analyzed for their cation content by capillary electrophoresis (CE). Cation extraction was conducted as follows: plant tissues were homogenized in liquid nitrogen and mixed thoroughly for 20 min in Milli-Q water (10 μL per 1 mg of tissue powder). The samples were centrifuged at 17730 × g for 5 min, and the resultant supernatants were collected and analyzed on a CE1600 (Hewlett Packard, Palo Alto, CA, USA) system using a Cation Solution Kit (Agilent, Santa Clara, CA, USA), according to the manufacturer’s instructions. The concentrations of five cations (NH4+, K+, Na+, Ca2+, and Mg2+) were measured.
Statistical analysisA Statistical analysis was conducted for the comparison of cation concentrations. For Experiments 1 and 2, a t-test was used to compare the two cultivars at each FTA. To compare several experimental sections in Experiment 3, the Tukey–Kramer test was employed, setting the significance value at P < 0.05.
RNA isolation and real-time PCRThe samples used for total RNA extraction were identical to those used for cation measurement. Total RNA was extracted as described by Ueno et al. (2012) with an additional DNase treatment. The concentration of the extracted RNA was determined using a Nanodrop spectrophotometer (Thermo Scientific, Waltham, MA, USA). Real-time PCR was conducted with the THUNDERBIRD SYBR qPCR Mix (TOYOBO, Osaka, Japan), using cDNA synthesized with a QuantiTect Reverse Transcription Kit (QIAGEN, Hilden, Germany). The primers used in this study were designed using the QuantPrime web-based tool (Arvidsson et al., 2008), except for the Ubiquitin primers which were taken from Jiang et al. (2013). The DNA sequences of the primers used in this study are listed in Table S2.
Firstly, to address the emerging pattern of leaf marginal necrosis, CMJ and RYO were grown. In this experiment, the anthesis of the 3rd to the 6th fruit truss was observed around September 25, October 1, October 9, and October 21, respectively. Although the plant size was slightly higher in CMJ than in RYO, the fruit and leaf numbers were almost the same in the two cultivars, indicating that their growth was mostly the same (Fig. 1A, B). However, the symptom intensity of the two cultivars was distinctly different, with necrosis observed only in CMJ (Fig. 1C). The symptoms began to appear at the 5th FTA and were frequently observed around the 6th FTA. Additionally, the symptoms were apparent in the tip leaflet of each compound leaf, and never appeared at the base leaflet during the experiment (data not shown).

Growth data for the tomato ‘CF Momotaro J’ (CMJ) and ‘Reiyo’ (RYO) from Experiment 1. (A) Fruit and (B) leaf numbers, observed at from the 3rd to the 6th fruit truss anthesis (FTA). (C) Symptom intensity of leaf marginal necrosis is shown by a box plot. The values of the maximum, third quartile, and median were 1 for CMJ at the 5th FTA. Three replicates were taken for each sample, and the error bars represent the standard deviations for (A) and (B).
Since previous study suggested that the K+ insufficiency plays a role in the development of leaf marginal necrosis, the tip (upper and lower compound leaf of the flowering fruit truss) and base leaflets (lower compound leaf of the flowering fruit truss) were collected based on the flowering fruit truss, from the 3rd to the 6th FTA, and the cation concentrations were measured and compared between the two cultivars (Fig. 2). No notable difference was observed for NH4+, Na+, Ca2+, and Mg2+ between the two cultivars and positions measured (data not shown). Between the two cultivars, RYO tended to show 30 to 40% higher K+ concentration in the tip leaflets of the lower compound leaf of the flowering fruit truss after the 4th FTA, although the difference was not statistically significant (P < 0.1), probably due to the small sample size (n = 3) and large variance within the replicates. Since leaf marginal necrosis is often observed at the leaf margins, it was assumed that the difference between the two cultivars may have been weakened by using the entire tip leaflet for analysis. Therefore, in the subsequent experiment, the leaves were analyzed after their separation into tip and basal regions.

Measured K+ content of leaflets around the flowering fruit truss, of ‘CF Momotaro J’ (CMJ) and ‘Reiyo’ (RYO) during Experiment 1 are represented. Samples were collected from the 3rd to the 6th fruit truss anthesis (FTA). Three replicates were taken for each sample. The error bars represent the standard deviation of the measurements and the asterisks represent the results of a t-test: significant at P < 0.1.
In addition to the two cultivars employed in Experiment 1, MoG and RNK, which exhibit leaf marginal necrosis frequently and rarely, respectively, (as reiterated in the handling manuals of both cultivars), were used. During cultivation, leaf marginal necrosis was first observed in MoG at the 2nd FTA, and was frequently observed together with CMJ at the 4th FTA (Fig. 3A). No symptoms were visible in the RYO and RNK. Based on this result, the CMJ and MoG were defined as the sensitive cultivars in Experiment 2.

Symptom and K+ data of the tomato ‘Reiyo’ (RYO), ‘Rinka409’ (RNK), ‘Momotaro grande’ (MoG), and ‘CF Momotaro J’ (CMJ) during Experiment 2. (A): Symptom intensities at the flowering of the 4th fruit truss are represented as a box plot. (B–E): The K+ content of the basal and tip regions of the leaflet samples at the flowering of the 3rd fruit truss are shown. Gray bars represent the basal region sample, and white bars represent the tip region sample. Three to four replicates (except for RYO, which only had two replicates) were taken for each sample. The error bars represent the standard deviation of the measurements and the asterisks above the bars show significant differences (t-test, P < 0.05 and P < 0.01).
To examine whether the K+ concentrations of the tip and basal regions differed from each other, and related to leaf marginal necrosis, the leaflet samples were collected at the 3rd FTA, after the first symptom of leaf marginal necrosis was observed (collected samples showed no symptoms), and were analyzed for their cation concentrations. For all cultivars, the tip region tended to show lower K+ concentration compared to the basal region, although this difference was not significant with the RYO and RNK, which did not show any symptoms of leaf marginal necrosis during cultivation (Fig. 3B, C). However, a significant difference was observed for the two sensitive cultivars, of which the tip regions only had approximately 50% of the K+ concentration observed at the basal region (Fig. 3D, E). This data suggests the possibility that the tip region of a leaflet is under K+ starvation in sensitive cultivars.
Previously, several studies have reported that the jasmonate-related genes are upregulated in the K+-deficient conditions in the model plant A. thaliana (Armengaud et al., 2004; Cao et al., 2006; Troufflard et al., 2010). Since sensitive cultivars showed low K+ concentration in the tip region of leaflets, the expression of the jasmonate-responsive LOXD gene was analyzed to clarify if the K+ starvation reaction observed in A. thaliana was also activated in the tomato. Not only K+ starvation, but also other stress signals are also known to induce leaf chlorosis. Since most abiotic stress signals are reported to be mediated by abscisic acid, ethylene, and jasmonates (Jibran et al., 2013; Zhang et al., 2011), the abscisic acid- and ethylene-responsive genes, SlRD22 and Chi9, respectively (Kissoudis et al., 2017) were additionally examined to determine if these abiotic stress signals were activated (Fig. 4). When the basal and tip regions of the leaflet were compared, the expression level of the jasmonate-responsive LOXD was upregulated in the tip region for both cultivars, while the abscisic acid-responsive SlRD22 was rarely different between the two cultivars, and the ethylene-responsive Chi9 was different only in the MoG. To further confirm that the possible jasmonate response is related to the K+ concentration, the relative gene expression values of several jasmonate-related genes were compared against the K+ concentration of each individual sample. As shown in Figure 5, the expression levels of the jasmonate-related genes—biosynthesis gene LOXD and SlAOS (Fig. 5A, B) and jasmonate-responsive gene SlJAZ1 (Fig. 5C)—were found to increase in the samples with low K+ concentrations. Additionally, a homolog of SAG12 (which is used as a molecular marker of leaf senescence in A. thaliana and is applicable to the tomato (Lira et al., 2014; Weaver et al., 1998) was also tested, and was detected in only four samples with the lowest K+ concentrations (Fig. 5D). Previously, several groups have reported that jasmonate could induce, and is involved in leaf senescence (Cao et al., 2006; Guo, 2013; He et al., 2002; Woo et al., 2013; Wu et al., 2008). Taken together, these data suggest that i) the low K+ concentration in the leaf tip regions activated jasmonate-responsive signals and induced the expression of jasmonate-related genes, ii) particularly, the strongly activated jasmonate-responsive signals further induced leaf senescence in severe K+ starvation conditions. This hypothesis matches the fact that the early symptoms of leaf marginal necrosis are similar to leaf senescence. To further confirm the association of jasmonate-responsive signals induced by K+ starvation with the occurrence of leaf marginal necrosis, an additional test was conducted to investigate the effect of an additional K+ supplementation in the growth medium.

Gene expression values of the basal and tip regions at the flowering of the 3rd fruit truss of (A): ‘Momotaro grande’ (MoG) and (B): ‘CF Momotaro J’ (CMJ) leaflets during Experiment 2. The relative expression values (ΔCT values) of LOXD, SlRD22, and Chi9 were calculated using Ubiquitin as a reference. Three replicates were taken for each sample, and the error bars represent the standard deviation of the measurements.

Comparison of the expression levels of the jasmonate-related (A–C) and leaf senescence-associated (D) genes against K+ concentrations of the basal and tip region of the tip leaflet from ‘CF Momotaro J’ (CMJ) and ‘Momotaro grande’ (MoG) in Experiment 2. The relative expression value (ΔCT value) of each gene was calculated using Ubiquitin, and plotted against K+ concentration of each individual sample. For SlSAG12, the gene expression was detected only in the four samples collected from the tip region. The black circles represent the samples from the basal region of a tomato leaflet, and the open circles represent the samples collected from the tip region.
In Experiment 3, the occurrence of leaf marginal necrosis was monitored under cultivation conditions with additional K+ supplementation using CMJ and RYO. The symptoms of leaf marginal necrosis were first observed at the 5th FTA in the control medium, followed by the occurrence at the 6th and the 8th FTA in the 8K and 6K media (Fig. 6). In this experiment, the symptoms of leaf marginal necrosis were also observed in RYO at the 8th FTA. Similar to Experiment 1, the symptom intensity tended to become severe as the FTA increased.
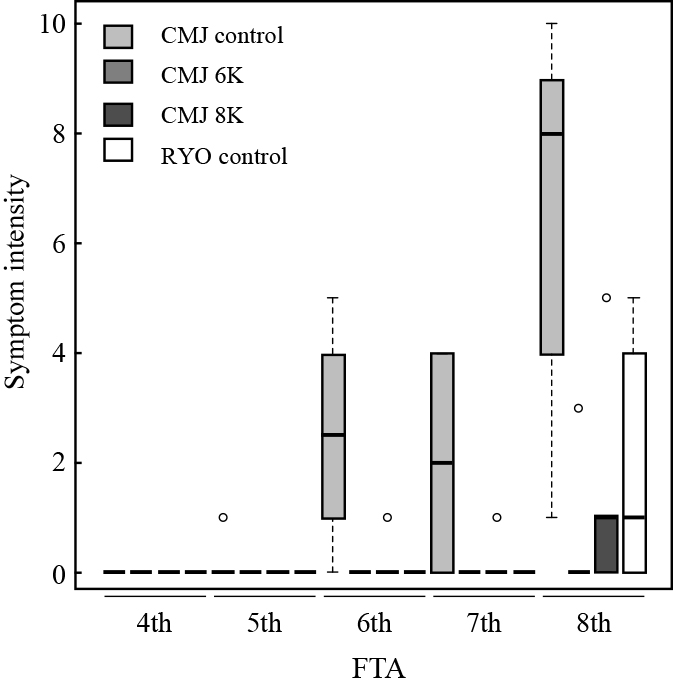
Symptom intensities of the ‘CF Momotaro J’ (CMJ) and ‘Reiyo’ (RYO) grown in the control medium and CMJ grown in the media with various K+ concentrations from the 4th to the 8th fruit truss anthesis (FTA) in Experiment 3 are represented. Control indicates the plants grown in control medium, 6K and 8K represent the plants grown in 6 me·L−1 and 8 me·L−1 K+-supplemented media, respectively.
To clarify whether K+ supplementation affected the ionic balance in tomato leaves, the cation content of the tip leaflet positioned just under the flowering fruit truss was analyzed at the 4th FTA (Fig. 7A, B). The K+ concentration was higher in the CMJ plants grown in the 6K and 8K media compared to the plants grown in the control medium, while Ca2+ showed the opposite trend. The K+ concentrations of the RYO plants were similar to those of the CMJ control plants at the 4th FTA. This result indicated that an increase in the K+ concentration of the growth medium led to an increase in the K+ concentration of the CMJ leaflets, consequently reducing the symptoms of leaf marginal necrosis.

Cation concentration and gene expression of the tip leaflet sample of the tomato ‘CF Momotaro J’ (CMJ) and ‘Reiyo’ (RYO) collected during Experiment 3 are represented. (A) K+ and (B) Ca2+ concentration at the flowering of the 4th fruit truss are shown. Different letters above the bars show significant differences (Tukey-Kramer test, P < 0.05). The relative expression values (ΔCT values) of (C) LOXD, (D) JAZ1 calculated using Ubiquitin as a reference at the flowering of the 4th fruit truss are shown. Three replicates were taken for each sample. The error bars represent the standard deviation of the measurements. Control indicates the plants grown in control medium, and 6K and 8K represent the plants grown in the K+-supplemented 6K and 8K media, respectively.
The result of Experiment 2 indicated that K+ starvation activated jasmonate signals. Since the K+ concentration in the tip leaflet differed between the plants grown in the control and K+-supplemented media, the investigation of jasmonate-responsive gene expression was conducted to provide further confirmation. As expected, the expression of jasmonate-responsive genes was lower in the tip leaflet of plants grown in higher K+ concentrated medium (Fig. 7C, D). Along with the results of Experiments 1 and 2, these data suggest that a decrease in K+ concentration in tomato leaflets plays an important role in the occurrence of leaf marginal necrosis, and an additional supplementation of K+ in the growth medium could reduce the symptoms of this disorder by compensating the decrease in K+ concentration in the tip region of tomato leaflets. However, in this experiment, the leaf K+ content and symptoms of leaf marginal necrosis were almost the same in CMJ grown in the 6K and 8K media (Figs. 6 and 7), suggesting an additional supplementation of K+ to the nutrient solution is not sufficient to further reduce the symptoms of leaf marginal necrosis. Additionally, compared to the control plants of the CMJ, the RYO was found to show a lower expression of jasmonate-responsive genes, indicating a difference in the K+ concentration response between the two cultivars, which may be related to the difference in the occurrence of leaf marginal necrosis. This difference in the K+ concentration response between the two cultivars may lead to a difference in the sensitivity to leaf marginal necrosis, although further study will be required to confirm this hypothesis.
ConclusionResults from the three experiments showed that a reduction in the K+ concentration in the tip region of a tomato leaflet plays an important role in the occurrence of leaf marginal necrosis, and that a jasmonate signal is involved in the response to low K+ concentration in the tip region of a leaflet. These results suggest the possibility that the expression levels of jasmonate-related genes could be a guidepost for research into the optimum cultivation conditions for the tomato, and minimizing the occurrence of this physiological disorder.