Abstract
We investigated the resistance to stem blight disease (Phomopsis asparagi (Sacc.)) in the progeny of two combinations of interspecific crosses between Asparagus officinalis (sensitive) and Asparagus A. kiusianus (resistant) in an effort to produce resistant cultivars. The progeny showed different degrees of disease severity, depending on the combination of crosses. Most of the hybrids derived from AO0060 (A. officinalis) × AK0501 (A. kiusianus) showed high disease resistance comparable to that of A. kiusianus. The results indicate that disease resistance could be introduced from A. kiusianus into A. officinalis, and that the selection of an appropriate cross combination is important for the production of disease-resistant cultivars. We analyzed the parents and hybrids of reciprocal crosses between A. officinalis and A. kiusianus using derived cleaved amplified polymorphic sequence markers to investigate the inheritance of the chloroplast genome, whose inheritance and genetic characteristics are not yet known. The chloroplast DNAs were inherited from the maternal parent, indicating that no major genes related to stem blight resistance were found in the chloroplast DNA.
Introduction
Stem blight in asparagus (Asparagus officinalis L.) caused by Phomopsis asparagi (Sacc.) is a disease that kills the host plants and substantially reduces crop production in many countries, including the USA, Australia, New Zealand, China, Greece, and Brazil (Davis, 2001; Elena, 2006; McKirdy et al., 2002; Reifschneider and Lopes, 1982; Udayanga et al., 2011). Primary infection occurs via soil. In plants infected by P. asparagi, small lesions or spots initially form on the stem surface a short distance above the ground. As the lesions expand, pycnidia usually appear in the central part of the lesion and become secondary sources of infection (Sakai et al., 1992; Sonoda et al., 1997). Infected stems eventually wilt, and the plant dies.
In Japan, asparagus is widely cultivated, and stem blight is a serious problem in almost all production areas, especially in open-field culture in warm regions. In these regions, asparagus is grown under shelter from the rain, and chemical control is used; these difficult and expensive measures are the main solutions to avoid this disease (Kobayashi and Shinsu, 1990). Breeding of resistant cultivars is, therefore, urgently required. Sonoda et al. (1997, 2001) reported that several hermaphroditic species in the genus Asparagus (which includes 100 to 300 species), such as A. densiflorus, A. virgatus, A. asparagoides, and A. macowanii, have strong resistance to stem blight, although all commercial A. officinalis cultivars are sensitive.
Although interspecific hybrids offer one way to improve resistance of A. officinalis cultivars, crosses between resistant wild species and A. officinalis have not been obtained because of the distant relationship among members of this genus (Sonoda et al., 2001). Interspecific hybrids, produced by cell fusion technique using electro pulsation from protoplasts of A. officinalis and A. macowanii, did not grow into adult plants, since they might have an abnormal genome composition (Kunitake et al., 1996). A. kiusianus Makino originating from the restricted Japan’s coastal regions, along the Sea of Japan from Yamaguchi Prefecture to Northern Kyushu, is a dioecious species like A. officinalis (Makino, 1907) (Fig. 1). Iwato et al. (2014) reported that A. kiusianus had stronger resistance to stem blight than A. officinalis, and Ito et al. (2011) found that production of hybrids from A. officinalis and A. kiusianus is possible. Iwato et al. (2014) also reported that individuals among hybrids between the two species varied in the strength of their disease resistance, and that some had resistance similar to that of A. kiusianus. Thus, in the present study, one of our goals was to investigate the inheritance of disease resistance in the progeny of interspecific crosses between A. officinalis and A. kiusianus.

Simple sequence repeat markers, which could be used to detect polymorphisms in the nuclear genome between A. officinalis and A. kiusianus, have already been examined (Nakatate et al., 2016), but there have been no reports about polymorphic markers for chloroplast DNA (cpDNA). Although cpDNAs are generally inherited from the maternal parent in angiosperms (Corriveau and Coleman, 1988), some reports have documented a surprising amount of variation in inheritance patterns, suggesting that cpDNA could be maternally or paternally inherited, as in Medicago (Forsthoefel et al., 1992) and in Actinidia (Jung et al., 2003; Testolin and Cipriani, 1997), or paternally inherited in interspecific crosses as in the Passifloraceae (Hansen et al., 2007). cpDNA inheritance in interspecific hybridization between A. officinalis and A. schoberioides was shown to be maternal by using restriction-fragment-length polymorphism analysis (Ito et al., 2007), but the mechanism of inheritance between A. officinalis and A. kiusianus remains unknown. A second objective of this study was, therefore, to resolve the inheritance of cpDNA in hybrids between A. officinalis and A. kiusianus to provide insights into the possible involvement of cpDNA genes in stem blight resistance.
Materials and Methods
Inoculation test
Two interspecific crosses, AO0042 × AK0102 and AO0060 × AK0501, between A. officinalis ‘Mary Washington 500W’ (the AO accessions) as the female parent and A. kiusianus (the AK accessions) as the male parent, were performed at Tohoku University, Japan. The parents, three hybrids of AO0042 × AK0102, and eight hybrids of AO0060 × AK0501, were used for inoculation tests. Four A. officinalis accessions (AO-1, AO-2, AO-4, and AO-6) and three A. kiusianus accessions (AKN-2, AKN-4, and AKN-6) were also tested to compare the resistance among populations of A. officinalis, A. kiusianus, and hybrids.
The inoculation test was conducted at Kyushu University from October to November 2012. Phomopsis asparagi strain P1, obtained from the Saga Prefectural Agricultural Experiment Station (Saga, Japan), was cultured on potato sucrose agar medium at 25°C. After sporulation, we prepared a spore suspension adjusted to an inoculum density of 2 × 106 spores·mL−1. Inoculation of P. asparagi and evaluation of disease resistance to stem blight followed the methods described by Iwato et al. (2014). In summary, 4- to 6-year-old plants were propagated by division and planted in pots to allow control of temperature, light intensity, and humidity in a growth chamber. Shoots of these plants were cut back to ground level to promote new shoot emergence. Newly emerged shoots (one per individual), 2 or 3 weeks after cutting, were inoculated. The main stems were wrapped with cotton that had been soaked in the spore suspension at a height of 2 to 5 cm above the lowest branching node, and were then covered with vinyl tape to prevent desiccation. The plants were incubated at 25°C and 90% relative humidity under 40000 lx light for 3 days after inoculation, and they were then kept at 25°C and 60 to 70% relative humidity under 40000 lx in a growth chamber after removal of the cotton and vinyl tape. Disease severity was determined weekly for 5 weeks as a disease severity grade (DSG): 0 = no lesion; 1 = small lesion (<1 cm); 2 = spread lesion; 3 = large lesion (>half the plant) or defoliation; and 4 = death of the aerial parts.
Identification of cpDNA polymorphism
DNA was isolated from young cladophylls of A. officinalis ‘Welcome’ (WC-114L) and A. kiusianus (Nijinomatsubara line AkN-1m) planted in Fukuoka, Japan. It was extracted by the modified CTAB method (Stajner et al., 2002). The DNA concentration was adjusted to approx. 20 ng·μL−1. Polymerase chain reaction (PCR) amplification of the rbcL region of the cpDNA was performed in a total volume of 50 μL containing 48 ng of template DNA, 0.5 μM forward and reverse primers, 5 μL of 10× Taq polymerase PCR buffer (Takara, Kusatsu, Japan), 0.2 mM of each dNTP (Takara), and 1.25 U Takara Ex Taq HS polymerase (Takara). The primer sequence designs were based on a previous report (Shinozaki et al., 1986): rbcL_F, 5'-TTGGCAGCATTCCGAGTAA-3' and rbcL_R, 5'-TCTCCTAAAGTTCCTCCAC-3'.
Amplification was performed in a Takara PCR Thermal Cycler Dice TP-600 (Takara) with initial denaturation for 5 min at 95°C; 35 cycles of 1 min at 94°C, 1 min at 62°C, and 1.5 min at 72°C; and a final extension for 7 min at 72°C. rbcL fragments were subcloned into the pGEM-T Easy Vector (Promega, Madison, WI, USA) and transformed into competent high Escherichia coli DH5α (Toyobo, Osaka, Japan). After culture on LB plates containing 100 μg·mL−1 ampicillin, 100 μg·mL−1 X-Gal, and 23.83 μg·mL−1 isopropyl-β-d-thiogalactopyranoside, target clones were selected, and then plasmids containing the inserts were extracted using a LaboPass Plasmid Mini Purification Kit (Hokkaido System Science, Sapporo, Japan).
Sequences were analyzed with a BigDye Terminator v. 1.1 Cycle Sequencing Kit (Applied Biosystems, Tokyo, Japan) and an ABI PRISM 310 genetic analyzer (Applied Biosystems). rbcL sequences were aligned in v. 3.2 of DNASIS software (Hitachi Software Engineering, Tokyo, Japan) to detect single-nucleotide polymorphisms (SNPs). We examined whether the SNPs could be converted into CAPS markers. If not, then we designed dCAPS primers. PCR of two regions (rbcL_A and rbcL_B) that included a SNP was conducted with initial denaturation for 5 min at 94°C; 35 cycles of 1 min at 94°C, 1 min at 40°C (rbcL_A) or 42°C (rbcL_B), and 1.5 min at 72°C; and a final extension for 15 min at 72°C. We then digested the PCR products (10 μL) in 2.0 μL of CutSmart buffer (New England Biolabs, Ipswich, MA, USA) and 1.0 μL (5 U) of AclI restriction enzyme (New England Biolabs) (rbcL_A) or 0.5 μL (10 U) of SacI-HF restriction enzyme (New England Biolabs) (rbcL_B) for 3 h at 37°C. The digested DNA was electrophoresed in 3.0% agarose gels, which were stained with Midori Green Advance DNA Stain Solution (Nippon Genetics, Tokyo, Japan), and visualized under LED 100 illumination (AMZ System Science, Osaka, Japan).
Inheritance of cpDNA
Reciprocal crosses between A. officinalis and A. kiusianus were made at Tohoku University and Kyushu University in Japan (Table 2). The parents and offspring were used for investigation of their cpDNA haplotypes. DNA extraction and confirmation of polymorphism were performed as described in the previous section.
Results and Discussion
Inoculation test
The aerial parts of A. officinalis died 3 or 4 weeks after inoculation, whereas A. kiusianus survived (Table 1). In A. kiusianus, the symptoms of stem blight did not spread, although symptoms appeared on the stems within 1 week after inoculation. These results agree closely with those of Iwato et al. (2014). The progeny of each cross combination showed different degrees of disease resistance even when A. kiusianus strains with similar degrees of the resistance were used as the pollen parents. The DSGs of all of progeny of AO0042 (A. officinalis) × AK0102 (A. kiusianus) resembled those of the female parent, since the aerial parts of two individuals were dead and one had a large lesion that covered more than half of the plant (Table 1). No hybrids showed strong disease tolerance in this cross combination. On the other hand, most of the eight hybrids of AO0060 (A. officinalis) × AK0501 (A. kiusianus) had high disease resistance similar to that of their pollen parent, A. kiusianus (Table 1). They formed only a small lesion by 5 weeks after inoculation. The hybrids obtained from the cross with AK0501 had stronger disease resistance than the hybrids from the cross with AK0102, indicating that AK0501 has greater potential as a pollen parent to produce disease-resistant cultivars. The difference in disease resistance between the two cross combinations might be caused by several factors, including the high heterozygosity of A. kiusianus and polygenic inheritance of the resistance trait. These results suggest that the strong disease resistance of A. kiusianus can be introduced into A. officinalis using resistant accessions of A. kiusianus, and that selection of parents whose progeny expressed strong resistance will be necessary for the breeding of disease-resistant cultivars.

Sequence analysis revealed two SNPs, at 40 bp (T/C) and 136 bp (C/A), in WC-114L/AkN-1m (Fig. 2). As there were no restriction enzyme recognition sites at the SNPs, we tried to introduce sites by adding a 1-bp substitution (T–C) in the forward primer of rbcL_A and another (A–G) in that of rbcL_B for AclI and SacI-HF digestion, and successfully converted the SNP marker into a dCAPS marker (Fig. 2). The sizes of the PCR products were 280 bp in rbcL_A and 268 bp in rbcL_B in both WC-114L and AkN-1m (Fig. 3A). Amplicons of rbcL_A and rbcL_B could be digested by AclI and SacI-HF, respectively, only in A. officinalis (WC-114L); that is, the 250-bp fragment digested from the 280-bp amplicon in rbcL_A and the 238-bp fragment digested from the 268-bp amplicon in rbcL_B were recognized in WC-114L (Fig. 3B). The 280-bp fragment in rbcL_A and the 268-bp fragment in rbcL_B were not digested with the restriction enzymes in A. kiusianus (AkN-1m). Thus, cpDNA polymorphism between A. officinalis (WC-114L) and A. kiusianus (AkN-1m) could be identified by using the dCAPS marker.

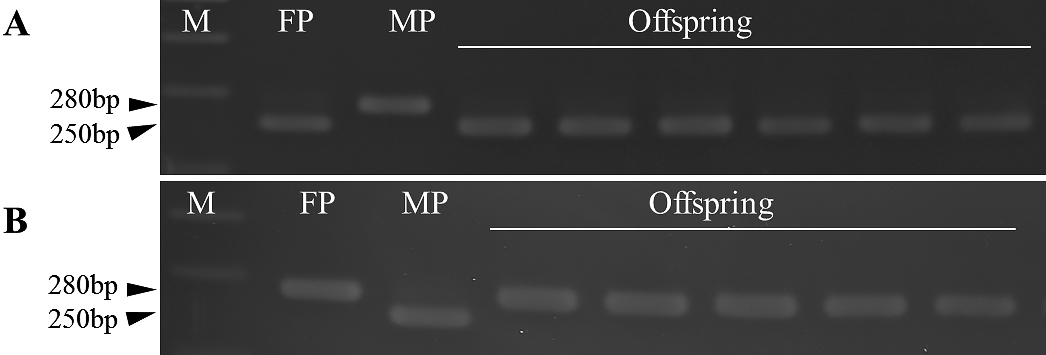
PCR products of 280 bp (rbcL_A) and 268 bp (rbcL_B) were identified in all of the parents and their progeny. Polymorphism between A. officinalis and A. kiusianus was confirmed in both rbcL_A and rbcL_B in all cross combinations by means of dCAPS analysis (Table 2; Fig. 4). We found haplotypes of 250 bp in rbcL_A and 238 bp in rbcL_B in A. officinalis, but those of 280 bp in rbcL_A and 268 bp in rbcL_B in A. kiusianus. The hybrids obtained from the four interspecific reciprocal crosses between A. officinalis and A. kiusianus showed the same dCAPS haplotypes as their female parents, indicating maternal inheritance in the interspecific crosses.

We found that stem blight resistance can be introduced from A. kiusianus into A. officinalis by interspecific crosses, and that the cpDNA of the hybrids of AO0060 (A. officinalis) × AK0501 (A. kiusianus), which had disease resistance similar to that of A. kiusianus, was the same as in AO0060 (A. officinalis). These results suggest that no major genes related to stem blight resistance were present in the cpDNA.
Literature Cited
- Corriveau, J. L. and A. W. Coleman. 1988. Rapid screening method to detect potential biparental inheritance of plastid DNA and results for over 200 angiosperm species. Am. J. Bot. 75: 1443–1458.
- Davis, R. D. 2001. Asparagus stem blight recorded in Australia. Australas. Plant Pathol. 30: 181–182.
- Elena, K. 2006. First report of Phomopsis asparagi causing stem blight of asparagus in Greece. Plant Pathol. 55: 300.
- Forsthoefel, N. R., H. J. Bohnert and S. E. Smith. 1992. Discordant inheritance of mitochondrial and plastid DNA in diverse alfalfa genotypes. J. Hered. 83: 342–345.
- Hansen, A. K., K. E. Linda, E. G. Lawrence and K. J. Robert. 2007. Paternal, maternal, and biparental inheritance of the chloroplast genome in Passiflora (Passifloraceae): implications for phylogenetic studies. Am. J. Bot. 94: 42–46.
- Ito, T., I. Konno, S. Kubota, T. Ochiai, T. Sonoda, Y. Hayashi, T. Fukuda, J. Yokoyama, H. Nakayama, T. Kameya and A. Kanno. 2011. Production and characterization of interspecific hybrids between Asparagus kiusianus Makino and A. officinalis L. Euphytica 182: 285–294.
- Ito, T., T. Ochiai, H. Aizawa, T. Shimodate, T. Sonoda, T. Fukuda, J. Yokoyama, T. Kameya and A. Kanno. 2007. Production and analysis of reciprocal hybrids between Asparagus officinalis L. and A. schoberioides Kunth. Genet. Resour. Crop Evol. 54: 1063–1071.
- Iwato, M., M. Kosaza, Y. Takeuchi, M. Matsumoto, M. Inada, Y. Ozaki and H. Okubo. 2014. Stem blight resistance of Asparagus kiusianus and its hybrid with A. officinalis. Adv. Hortic. Sci. 28: 202–207.
- Jung, Y. H., S. C. Kim, M. Kim, K. H. Kim, H. M. Kwon and M. Y. Oh. 2003. Chloroplast inheritance patterns in Actinidia hybrids determined by single stranded conformation polymorphism analysis. Mol. Cells 15: 277–282.
- Kobayashi, M. and T. Shinsu. 1990. Rain shelter cultivation of green asparagus. Bull. Nagasaki Agric. For. Expt. Sta. 18: 117–145.
- Kunitake, H., T. Nakashima, K. Mori, M. Tanaka, A. Saito and M. Mii. 1996. Production of interspecific somatic hybrid plants between Asparagus officinalis and A. macowanii through electrofusion. Plant Sci. 116: 213–222.
- Makino, T. 1907. Observation on the flora of Japan. Bot. Mag. Tokyo 21: 161–163.
- McKirdy, S. J., P. Murphy, A. E. Mackie and S. Kumar. 2002. Survey of asparagus in Western Australia for rust and stem blight. Australas. Plant Pathol. 31: 97–98.
- Nakatate, C., E. Kakizoe, S. Yamamoto, M. Iwato, T. Matsuishi, K. Tomiyoshi and Y. Ozaki. 2016. Selection of co-dominant SSR markers applicable in interspecific crossing between Asparagus officinalis and A. kiusianus. Int. Symp. Agricultural, Food, Environmental and Life Sciences in Asia.
- Reifschneider, F. J. B. and C. A. Lopes. 1982. Phoma asparagi on asparagus. FAO Plant Prot. Bull. 30: 157.
- Sakai, Y., T. Ito and A. Tanaka. 1992. The primary source of stem blight of asparagus (Asparagus officinalis L.) and the disease spread from them. Bull. Hiroshima Pref. Agr. Res. Ctr. 55: 97–107.
- Shinozaki, K., M. Ohme, M. Tanaka, T. Wakasugi, N. Hayashida, T. Matsubayashi, N. Zaita, J. Chunwongse, J. Obokata, K. Yamaguchi-Shinozaki, C. Ohto, K. Torazawa, B. Y. Meng, M. Sugita, H. Deno, T. Kamogashira, K. Yamada, J. Kusuda, F. Takaiwa, A. Kato, N. Tohdoh, H. Shimada and M. Sugiura. 1986. The complete nucleotide sequence of the tobacco chloroplast genome: its gene organization and expression. EMBO J. 5: 2043–2049.
- Sonoda, T., A. Uragami, K. Itoh, H. Kohmura, M. Ohwada and K. Kaji. 2001. Evaluation of Asparagus species and comparison between sexes in A. officinalis cultivars for resistance to stem blight. J. Japan. Soc. Hort. Sci. 70: 244–250.
- Sonoda, T., A. Uragami and K. Kaji. 1997. Evaluation of Asparagus officinalis cultivars for resistance to stem blight by using a novel inoculation method. HortScience 32: 1085–1086.
- Stajner, N., B. Bohanec and B. Javornik. 2002. Genetic variability of economically important Asparagus species as revealed by genome size analysis and rDNA ITS polymorphisms. Plant Sci. 162: 931–937.
- Testolin, R. and G. Cipriani. 1997. Paternal inheritance of chloroplast DNA and maternal inheritance of mitochondrial DNA in the genus Actinidia. Theor. Appl. Genet. 94: 897–903.
- Udayanga, D., X. Liu, E. H. C. McKenzie, E. Chukeatirote, A. H. A. Bahkali and K. D. Hyde. 2011. The genus Phomopsis: biology, applications, species concepts and names of common phytopathogens. Fungal Diversity 50: 189–225.