2018 Volume 87 Issue 2 Pages 236-249
2018 Volume 87 Issue 2 Pages 236-249
The black soybean landrace ‘Tanbaguro’, consumed as vegetable seeds (edamame), is characterized by its uniquely high maltose production after heat processing, which is an important trait contributing to its enhanced sweetness. We investigated starch properties related to the maltose production in edamame together with several other affecting biochemical factors. As a result, it was estimated that the low gelatinization temperature of starch predominantly contributed to the increased maltose production and was associated with the increased proportion of short-chain amylopectin, although β-amylase activity did not show any significant correlation with maltose productivity. Additionally, amylose content may promote maltose production by the progression of starch gelatinization together with its effectiveness as a substrate of β-amylase. These starch properties were sensitive to ambient temperature, especially during the latter half of the maturation period. Therefore, it is suggested that maltose productivity in edamame seeds was strongly correlated with the maturing properties of soybean lines. On the other hand, starch content that was insensitive to ambient temperature during the maturing period was also estimated to contribute to the increase in maltose productivity complementarily by multiple regression analysis for varietal difference. These results suggested that starch content showed the additional importance in the varietal difference of maltose productivity among earlier maturing soybean lines than ‘Tanbaguro’.
The black soybean ‘Tanbaguro’ is a very late-maturing variety harvested through November until December, and has been used mainly as boiled beans. Meanwhile, consumption of ‘Tanbaguro’ as a vegetable soybean (edamame) harvested at an immature stage in mid-October has progressively increased (Hirota, 2014). Hirota et al. (2003) reported that late-maturing black soybeans, including ‘Tanbaguro’, were characterized by their large and soft seeds containing a higher amount of maltose after heat processing than ordinary edamame varieties. Vegetable seeds of ‘Tanbaguro’ and its relative lines after heat processing contain mainly sucrose and maltose (Furutani et al., 2012; Hirota, 2014; Hirota et al., 2003; Nomura et al., 2014), both of which contribute to their sweetness. However, maltose has an advantage over sucrose for stable sweetness owing to its rapid conversion after heat processing, while sucrose content rapidly decreases in raw edamame seeds after harvesting (Hirota et al., 2000, 2003; Mizuno et al., 2015). Formerly, several landraces that could be harvested earlier than ‘Tanbaguro’ were commonly cultivated in the Tanba region, Hyogo prefecture, to expand the cropping season of black soybean edamame. Recently, however, most of these landraces have discontinued cultivation because of the different taste due to the lower maltose content as compared with ‘Tanbaguro’. On the other hand, several earlier-maturing black soybean varieties have been bred in Hyogo prefecture and by other organizations. However, the amounts of maltose in these breeding varieties remain much lower than those in ‘Tanbaguro’ and its relative lines suitable for edamame, although the sucrose content in the raw seeds of these varieties is higher (Furutani et al., 2012; Hirota et al., 2010).
Maltose is not present in raw edamame seeds and is produced only after heat processing (Furutani et al., 2012; Hirota, 2014; Hirota et al., 2003; Masuda, 2003; Nomura et al., 2014). It is well known that in the sweet potato, a large amount of maltose is produced after cooking. Kiribuchi and Kubota (1976) reported that maltose was produced by the degradation of gelatinized starch with β-amylase in the sweet potato. It can be assumed that maltose in edamame is also produced by the same or a similar process. Soybean seeds, however, contain a much lower amount of starch compared with other beans. This may be one reason why no intensive studies have been conducted on the starch properties in relation to eating quality in soybean seeds.
It has been suggested that varietal differences in the gelatinization temperature of starch in edamame is associated with the maltose production ability (Masuda, 2004). Nomura et al. (2014) reported that ‘Shin-Tanbaguro’ exhibited a lower starch gelatinization temperature and a higher maltose content than leading edamame varieties. However, it remains largely unknown how the gelatinization temperature of starch can contribute to the maltose production ability in conjunction with several other factors, including ambient temperature during the ripening stage of edamame seeds.
In this study, we investigated the effects of starch properties possibly related to the maltose production ability in edamame using black soybean landraces that have discontinued production in the Tanba region at present, together with several other varieties exhibiting different maturing properties. We herein report our results of a re-evaluation of the relationship between these parameters and maltose production ability, together with possible effects of the ambient temperature during the seed-maturation period.
Fourteen black soybean lines, including improved varieties, used in this study are shown in Table 1 together with maturation characteristics and seed weights. They included 12 lines of black soybeans selected from landraces produced in Tanba and its neighboring regions (Hirota et al., 2012), and two improved varieties, ‘Satokkohime’ and ‘Kurokkohime’ as references, both of which were bred from the cross between ‘Wase-dadacha’ and ‘Hyoukei-kuro 3’ (Hirota et al., 2010). ‘Hyoukei-kuro 3’ is a standard line of ‘Tanbaguro’ in Hyogo prefecture. Soybean plants were grown under natural environmental conditions in an experimental field of the Hokubu Agricultural Institute, Hyogo, Japan in 2012. In the tested lines, maturation periods ranged from 50 to 75 days. Based on the maturation periods, the 14 soybean lines were classified into three groups in terms of maturation properties, i.e. medium (M), intermediate late (IL), and late (L) maturing lines, as shown in Table 1.

Maturation characteristics and seed weights in 14 black soybean lines used in this study together with mean ambient temperatures in the three maturation periods after flowering.
Based on the color change of the pods (an etiolation value of pods) according to Hirota et al. (2003), eighty pods of each line were harvested randomly from 10 plants, and the weights of intact immature seeds were recorded for each line. For heat treatment, pods were boiled at 100°C for five minutes. Both raw and boiled seeds were stored at −35°C until analysis.
Meteorological dataThe mean ambient temperature during the period from flowering to maturity for edamame in each soybean line was derived using AMeDAS (Automated Meteorological data Acquisition System) data for 2012 in Wadayama, Hyogo, Japan (Table 1). The three maturation periods studied for edamame were from flowering to the 30th day after flowering (F-30th), from the 31st day after flowering to optimum maturity for harvest (31st-M), and from flowering to optimum maturity for harvest (MP). The ambient temperatures during the maturation period for F-30th, 31st-M, and MP ranged from 26.5 to 27.3°C, 19.6 to 25.1°C, and 22.4 to 26.4°C among the investigated lines, respectively. These AMeDAS data were used in the correlation analysis with maltose production and starch properties.
Analysis of maltoseThe raw and boiled seed materials (5.0 g) were added to 15 mL of 80% ethanol, homogenized using an ultra-disperser, and extracted at room temperature for 1 h. After centrifugation at 3000 rpm for 10 min, the pellet was further extracted twice with 15 mL of 80% ethanol. The supernatant was collected, and the final volume was adjusted to 50 mL with 80% ethanol. The ethanol extract was filtrated through a 0.45 μm membrane filter (Advantec DISMIC-13JP; Toyo Roshi Kaisha Ltd., Tokyo, Japan). The filtrate was subjected to HPLC analysis using LC-9A (Shimadzu, Kyoto, Japan) equipped with a refractive index detector RID-6A (Shimadzu). Separation was carried out on a Shim-Pack CLC-NH2 column (6×150 mm; Shimadzu) by eluting with a mixture of acetonitrile and water (70:30, v/v) at 40°C at a flow rate of 1.0 mL·min−1. The concentration of maltose in the sample was calculated by comparing the peak areas of the maltose standard solution.
Analysis of total starch and amyloseAlcohol-insoluble solid (AIS) obtained as pellets after the extraction of maltose was dried and used for analysis of starch and amylose. Starch content was enzymatically determined using an amyloglucosidase/α-amylase method (McCleary et al., 1997) using a total starch assay kit (Megazyme International Irelands Ltd., Bray, Ireland).
Amylose content was enzymatically determined using an amylose/amylopectin assay kit (Megazyme International Irelands Ltd.) based on the concanavalin A method (Yun and Matheson, 1990).
Analysis of β-amylase activityEach raw seed sample (7.0 g) was homogenized in 100 mL of ice-cold acetone (−20°C) using an ultra-disperser, and filtered under aspiration. The pellet was washed with cold acetone and cold ethyl ether, and dried at room temperature. The acetone powder (0.5 g) obtained was added to 5 mL of 50 mM Tris-HCl buffer (pH 8.0), and extracted at room temperature for 1 h with frequent stirring. β-amylase activity was determined using a β-amylase Betamyl-3® assay kit (Megazyme International Irelands Ltd.) using the above extract (0.2 mL).
Determination of the thermal properties of starchThe weighed AIS sample (10 mg) was added to 500 μL of 2.5 mM HEPES-NaOH buffer (pH 7.4) containing 5 mM sodium chloride, 0.05 mM EDTA, and 0.0625% Triton X-100, and then sonicated 10 times for 5 min. The extract was added to a 65% sucrose solution, centrifuged at 12000 rpm for 20 min and the pellet was homogenized with a pestle. The pellet was resuspended in 100% acetone, centrifuged at 1200 rpm for 5 min and the final pellet was dried.
The weighed starch (3 mg) purified by the prior steps was placed in a silver cup, mixed with 25 μL of distilled water and sealed. Gelatinization properties of the starch were analyzed using a differential scanning calorimeter (DSC) (DSC6100; Seiko Instruments Inc., Tokyo, Japan). The heating rate was 1.5°C·min−1 over a temperature range from 30 to 120°C. Onset, peak, and conclusion temperatures of gelatinization (i.e. To, Tp, and Tc values, respectively) were determined as shown in DSC thermo-grams of the starch samples. Gelatinization enthalpy (ΔH) of the starch was obtained from the area of the endothermic peak surrounded by the baseline.
Determination of chain-length distribution of amylopectinThe AIS powder (5 mg) was suspended in 1.5 mL of methanol in a boiling water bath for 10 min and centrifuged at 3000 rpm for 5 min. The pellet was washed twice with 1.5 mL of 90% methanol. The dried pellet was added to 143 μL of distilled water and 7.5 μL of 5 N sodium hydroxide, vortex-mixed, and then boiled for 5 min. After the starch was gelatinized, the suspension was added to 4.8 μL of 100% acetic acid, 50 μL of sodium acetate buffer (pH 4.4), 7.5 μL of 2% NaN3, and 500 μL of distilled water. The sample was hydrolyzed with 3 μL (2 U) of Pseudomonas amyloderamosa isoamylase (ISA) (Hayashibara Biochem. Lab., Okayama, Japan) at 37°C overnight, incubated in a boiling water bath for 20 min and centrifuged. The supernatant was used as the ISA-treated sample. The ISA-treated glucan was deionized by filtration through an ion exchange resin in a microtube. The supernatant (50 μL) was evaporated to dryness in a centrifugal vacuum evaporator. The sample was dissolved in 2 μL of 25 mM 1-aminopyrene-3,6,8-trisulfonic acid trisodium salt (APTS) (Beckman Coulter, CA, USA) in 15% acetic acid and 2 μL of 40 mM sodium cyanoborohydride in tetrahydrofuran, and reacted at 55°C for 90 min. The reaction mixture was diluted to 10 times with distilled water prior to injection into the capillaries. After fluorescence labeling with APTS, the chain-length distributions of α-glucans from seeds were analyzed by the fluorophore-assisted carbohydrate capillary electrophoresis (FACE) method of O’Shea et al. (1998) using a P/ACE MDQ Carbohydrate System (Beckman Coulter). The amylopectin chain ratio on the basis of relative peak area was calculated by the method of Nakamura et al. (2002). Chain-length distribution was determined on the molar basis, and the peak area of a fraction of the linear chain with a specific chain length was calculated as a percentage of total peak area up to degree of polymerization (DP) of 60, while the fractions, i.e. fa (DP 6–12), fb1 (DP 13–24), fb2 (DP 25–36), and fb3 (DP>37), were classified according to the criteria proposed by Hanashiro et al. (1996).
Statistical analysisThe data were statistically analyzed by the Tukey-Kramer test with the level of significance set at P < 0.05 or 0.01. In addition, the correlation analysis and varietal differences analysis were performed for the starch properties.
Models of multiple regression were compared using Akaike Information Criteria (AIC) and determination coefficient values (R2). Namely, the explanatory variables in the multiple regression models were selected by the step-wise procedure where smaller AIC values indicated a better fit.
All statistical analyses involving correlation and multiple regression analysis were performed using the statistical computing program R (version 3.0.2).
Maturation properties of edamame in 14 black soybean lines are shown in Table 1. Mean harvesting dates of the three maturing groups, i.e. late (L), intermediate late (IL), and medium (M) maturing group were October 22, October 5, and September 21, respectively. Mean maturation periods differed significantly by more than 10 days among the three groups. Mean ambient temperatures were significantly different among the maturing groups with different maturation periods in 31st-M and MP, while in F-30th, that of only the L maturing group was lower than those of the other two groups. Seed weights ranged from 1.02 to 1.62 g among the tested lines. Mean seed weights of the three groups were 1.47 g for L, 1.27 g for IL, and 1.07 g for M, and significant differences were observed between the L and M maturing groups.
Maltose productionMaltose was not detected in the raw seeds before heat processing (data not shown). A scatter plot of maltose content after heat processing of 14 soybean lines in the three maturing groups is shown in Figure 1. Maltose content per 100 g dry weight (DW) of edamame seeds ranged from 1.04 to 3.62 g. ‘Hyoukei-kuro 3’ (L1) in the L maturing group had the highest maltose content of 3.62 g/100 g DW among the investigated lines. Mean maltose contents were 3.31 g in L, 2.59 g in IL, and 1.10 g in the M maturing groups respectively. Thus, the maltose production ability of the M maturing group was one-third lower than those of the L maturing group. As for the variation in the groups of soybean lines, maltose content of the IL maturing group exhibited a wide range of distribution from 1.96 g to 3.49 g, among which three lines (IL1, IL3, and IL4) contained equivalent amounts to those of the L maturing group, and the coefficient of variation (CV) of maltose contents in the IL maturing group was more than threefold higher than those of other maturing groups.
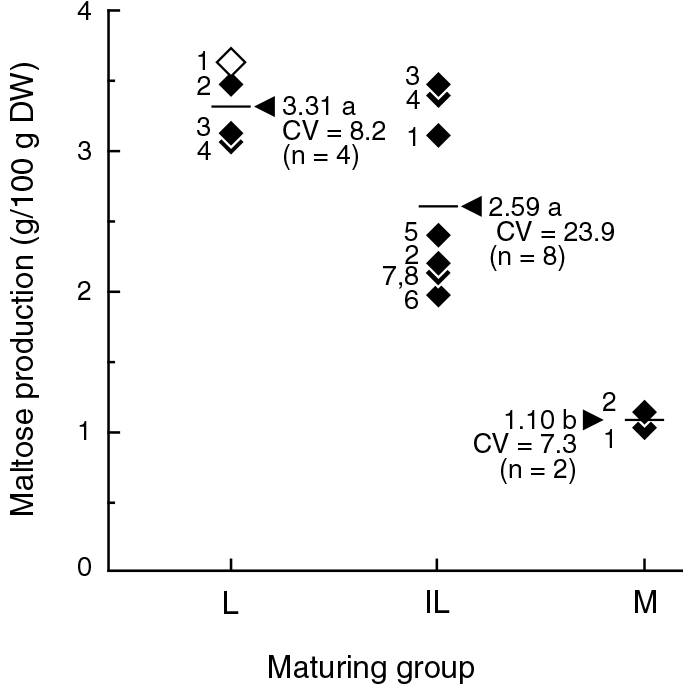
Scatterplot of maltose content in boiled seeds of 14 soybean lines. White diamonds indicate ‘Hyoukei-kuro 3’. Horizontal bars indicate means within line groups. Same letters to the right of the mean values indicate no significant differences between the means at the 5% level according to the Tukey-Kramer multiple comparison test. Numerical figures to the left of diamonds indicate the line numbers.
β-amylase activity, starch accumulation, and percentage of amylose content in raw seeds of 14 black soybean lines in the three maturing groups are shown in Table 2. β-amylase activity per 1 g DW of raw seeds ranged from 42.8 U to 73.8 U. Mean β-amylase activity was 61.9 U in L, 57.4 U in IL, and 49.5 U in the M maturing group, and no significant differences were observed among the three different maturing groups. Starch content per 100 g DW of raw seeds varied from 6.0 g to 12.8 g, but no significant differences were found among each group, although the mean value of the M maturing group was lower than those of other groups. Regarding the variation in the group of soybean lines, the coefficient of variation (CV) of starch contents in the IL maturing group was twice as large as that of the L maturing group, and the starch contents of IL3, IL4, and IL6 indicated either equaling or surpassing values for those of L1 and L2 especially in the L maturing group. The amylose content of raw seeds ranged from 11.1 to 17.4%. The mean amylose contents observed were 16.4%, 13.1%, and 11.9% in the L, IL, and M maturing groups, respectively. It was notable that the L maturing group contained a significantly higher amount of amylose than the other two groups.

β-amylase activity, starch, and amylose contents in raw seeds of 14 black soybean lines.
Starch gelatinization properties in the three maturing groups were investigated using DSC, and their mean starch gelatinization temperatures are shown in Table 3. DSC curves depicted by representative lines of the three maturing groups, i.e. a late maturing line ‘Hyoukei-kuro 3’ (L1), an intermediate late maturing line ‘IL-6’, and a medium maturing line ‘Satokkohime’ (M2) are shown in Figure 2. To, Tp, and Tc of starch were determined by the inflection temperature on a DSC thermogram. To, Tp, and Tc values in each maturing group increased from 57.1 to 64.7°C, from 60.5 to 70.2°C, and from 63.9 to 74.6°C in the direction of earlier maturing groups, respectively. Mean To, Tp, and Tc values, especially those of Tp and Tc in the L maturing group, were significantly lower than those in the other two maturing groups. The mean temperature range (Tc–To) of starch gelatinization was 6.8°C in L, 10.8°C in IL, and 9.9°C in the M maturing group. There was no clear comparison among the maturing groups. Although mean values of ΔH varied from 2.8 to 4.0 mJ·mg−1, there were no significant differences observed among the maturing groups.

The means of starch gelatinization characteristics in edamane seeds in three maturing groups measured by differential scanning calorimeter (DSC).

Differential Scanning Calorimeter (DSC) curves of seed starches in edamame. A, B, and C respectively show a DSC curve for a representative line of the three maturing groups, i.e., a late maturing line ‘Hyoukei-kuro 3’ (L1), an intermediate late maturing line (IL6), and a medium maturing line ‘Satokkohime’ (M2). Plus signs indicate onset (To), peak (Tp), and conclusion (Tc) temperatures of starch gelatinization.
Chain-length distribution patterns of amylopectin and the differences among ‘Hyoukei-kuro 3’ (L1), ‘IL6’, and ‘Satokkohime’ (M2) are shown in Figure 3. Amylopectin from ‘Hyoukei-kuro 3’ (L1) had enriched short chain fractions of DP 6–12, but lower proportions of intermediate chain fractions of DP 13–36 as compared with ‘Satokkohime’ (M2) and ‘IL6’. It was noted that this characteristic was more apparent when the comparison was made between ‘Satokkohime’ (M2) and ‘Hyoukei-kuro 3’ (L1) than between ‘IL6’ and ‘Hyoukei-kuro 3’ (L1). Patterns of differences in amylopectin chains in the three maturing groups, however, were very similar (data not shown). With respect to the chain-length distributions, amylopectin in 14 black soybean lines was classified into the following four fractions with different degrees of polymerization, i.e., fa with DP 6–12, fb1 with DP 13–24, fb2 with DP 25–36, and fb3 with DP>37, as shown in Table 4. Mean proportions of the fa, fb1, fb2, and fb3 fractions varied from 33.1 to 36.0%, from 53.8 to 55.6%, from 6.7 to 7.8%, and from 3.4 to 3.7%, respectively, in the three maturing groups. The proportion of the fa fraction was significantly higher in the L maturing group than in the other two groups. Unlike the fa fraction, the proportions of the fb1 and fb2 fractions tended to increase in the earlier maturing lines. Although proportions of the fb1 and fb2 fractions in the IL maturing group were in between those of the L and M maturing groups, each proportion of the fb1 and fb2 fractions in the IL maturing group was not significantly different from those in L and M maturing groups, respectively. Furthermore, no significant differences were found in the proportion of the fb3 fraction among different maturing groups.

Chain-length distribution of amylopectin in edamame seeds from representative lines of the three maturing groups. A and B respectively show a comparison of amylopectin chain-length distribution of an intermediate late maturing line (IL6) and a medium maturing line ‘Satokkohime’ (M2) with a late maturing line ‘Hyoukei-kuro 3’ (L1). C and D respectively show the difference in chain-length distribution of amylopectin between IL6 and LI and between M2 and L1. To determine chain-length distribution, see Materials and Methods.

The means of amylopectin chain-length distributions in edamame seeds of three maturing groups.
To study the putative effects of starch properties on the maltose production, correlation coefficients between maltose production and β-amylase activity, starch content, amylose content, gelatinization temperatures, and chain-length distribution of amylopectin in edamame seeds were calculated (Table 5). β-amylase activity demonstrated no correlation with maltose production. Starch and amylose contents showed positive correlations with maltose production, the correlation coefficients being higher with the amylose content (r = 0.786) than with the starch content (r = 0.541). To, Tp, and Tc values of starch gelatinization also demonstrated high and negative correlations with maltose production (r = −0.751, r = −0.842, and r = −0.814, respectively). In contrast, ΔH values representing gelatinization enthalpy demonstrated no correlations with maltose production. With respect to the chain-length distribution of amylopectin, the maltose production showed a strong positive correlation with the proportion of short chains in the fa fraction (r = 0.851) and negative correlations with the proportions of the fb1 and fb2 fractions (r = −0.780 and −0.643, respectively). Correlation diagrams showing relations between the maltose production and the three starch properties were compared (Fig. 4). In the edamame seeds after heat processing among all varieties, maltose production demonstrated a negative correlation with the Tp value, but positive correlations with proportions of the fa fraction in amylopectin and the amylose content. Although plots of maltose production appeared to be relatively linear for Tp value and the proportion of the fa fraction in amylopectin, for amylose content, lower maltose production appeared in the medium maturing lines than the expected values by linear regression.

Correlation coefficients between maltose content in edamame seeds and candidate characteristics related to maltose production.

Relationships between maltose production and starch properties in edamame seeds. Symbols indicate three maturing groups, i.e., black circles (L): late maturing lines (n = 4), grey triangles (IL): intermediate late maturing lines (n = 8), white squares (M), medium maturing lines (n = 2). Tp: gelatinization peak temperature, fa fraction: proportion of short-chain amylopectin (DP 6–12). r: correlation coefficient, **significance: P < 0.01.
We observed that the gelatinization temperatures exhibited a strongly negative correlation with the maltose production; thus, we further studied the possible relationships of other starch properties with maltose production through the effects on gelatinization characteristics (Table 6). Amylose content demonstrated strong negative correlations not only with gelatinization temperatures of To, Tp, and Tc, but also with the temperature range (Tc–To). In particular, the highest correlation was observed between amylose content and Tc value. The proportions of the amylopectin chain length were also correlated with gelatinization temperatures. The fa fraction demonstrated strongly negative correlations with To, Tp, and Tc values, while the fb1 and fb2 fractions showed high positive correlations either with To and Tp values or with Tp and Tc values, respectively, and showed especially high correlations with Tp values. The fb3 fraction, however, demonstrated no significant correlation with the gelatinization temperatures.

Correlation coefficients of starch properties and gelatinization temperatures of starch in edamame seeds.
Most starch properties were found to be significantly different among the maturing groups of soybean lines. Soybean lines with different maturation properties grew under different ambient temperatures during the maturation period. Therefore, we further studied possible effects of the ambient temperature conditions on maltose production and the starch properties in edamame seeds. Correlation coefficients among these parameters are shown in Table 7. The maltose production exhibited negative correlations with mean ambient temperatures in the three maturation periods of F-30th, 31st-M, and MP. Amylose content also demonstrated negative correlations with mean temperatures in the three maturation periods. In contrast, β-amylase activity and starch content showed no significant correlations with the ambient temperature. All three gelatinization temperature parameters showed strong positive correlations with the ambient temperature, especially in the second half period (31st-M) compared to the first half period (F-30th). With respect to the chain-length distribution of amylopectin, the short chain proportion of amylopectin in the fa fraction demonstrated negative correlations, while the chain proportion of amylopectin in the fb2 fraction showed positive correlations with the ambient temperature in all three maturation periods.

Correlation coefficients between candidate characteristics related to maltose production and mean ambient temperatures in the three maturation periods in edamame seeds.
The results of correlation analysis demonstrated that maltose production ability was related to the gelatinization temperature, which was associated with the distribution of amylopectin chain length and amylose content. In addition, these starch properties were strongly affected by ambient temperature conditions during maturation periods. However, the varietal differences in maltose productivity were not considered enough to account for the above starch properties because in several lines (IL1, IL3, and IL4) in the intermediate late maturing group, the same maltose production was observed relative to lines in the late maturing group. For this reason, complementary effects with other factors in addition to the above starch properties for maltose production were evaluated by multiple regression analysis. The explanatory variables in the multiple regression models were selected by the step-wise procedure with the evaluation of AIC (Table 8). As a result of this analysis, AIC in the regression model with the fa ratio in amylopectin as the initial explanatory variable was the lowest value (22.24), although the regression model with Tp was indicated as the second lowest AIC (23.03). Hence, the multiple regression models with secondary explanatory variables were evaluated with the fa ratio and Tp value. As a result, when the Tp value of initial explanatory variables was combined with starch content as the secondary variable in the multiple regression model, the AIC value had a greater decrease, to 5.54, than in combination with other secondary explanatory variables, although AIC values for the multiple regression models with the fa ratio as the initial explanatory variable ranged from 18.76 to 24.20 in combination with the secondary explanatory variables. However, the AIC values were not improved by the combination with third explanatory variables for the multi regression model with the Tp value and starch content. Consequently, the results of regression analysis are detailed in Table 9 for the single regression model with the Tp value, and the multiple regression model with the Tp value and starch content. The R2 and adjusted R2 for the multiple regression model with the Tp value and starch content were 0.928 and 0.914, respectively, and increased more than 0.708 and 0.684, respectively, for the single regression model with the Tp value. In the multiple regression model, t values of two explanatory variables were significant at the 1% level, respectively. Consequently, it was considered that the binary regression model with the Tp value and starch content best accounted for maltose productivity among soybean test lines. Additionally, it was considered that starch content was an important factor, along with Tp value, because standardized partial regression coefficients of Tp value and starch content were −0.800 and 0.470, respectively.

Akaike information criteria (AIC) values of the multiple regression model for maltose content with different numbers of explanatory variables.

The results of multiple regression analysis of maltose content for selected regression models.
Figure 5A and B show linear fitness between predicted and observed maltose contents according to a single regression model with Tp, and a multiple regression model with Tp and starch content. The multiple regression model provided a better prediction of maltose content than a single regression model, especially for soybean lines in the IL maturing group including IL1, IL3, and IL4 with a high maltose production ability.

A linear relationship between predicted and observed maltose content according to single (A) and multiple (B) regression models constructed using the gelatinization peak temperature (Tp) of starch (partial regression coefficient of X1:Tp) and the starch content (partial regression coefficient of X2:starch content) as explanatory variables in edamame seeds.
Raw starch has a micelle crystal structure that is bound by hydrogen bonds between amylose and amylopectin chains. Under this condition, plant β-amylase has low activity to digest raw starch (Ueda and Marshall, 1980). However, when raw starch was added to the water and heated, the starch crystal structure was dissolved and the starch granules swelled and gelatinized (Miura, 2003). Kiribuchi and Kubota (1976) and Walter et al. (1976) reported that the roasting of sweet potatoes resulted in a high conversion of starch into maltose, and as a result, heated and gelatinized starch became digestible with β-amylase. We therefore analyzed the relationship between β-amylase activity and maltose production after heat processing in edamame. However, we found no significant correlation between maltose production and β-amylase activity (Table 5) as already reported by other studies (Masuda, 2003, 2004).
Takahata et al. (1994) reported in sweet potatoes that the optimal temperature of β-amylase activity did not necessarily correspond with the starch gelatinization temperature, the latter being considerably higher than the former. Soybean β-amylase was reported to have a maximum activity at 60°C, and the T50 value, representing the temperature of 50% of the initial activity, was 63.2°C (Yoshigi et al., 1996). In edamame, the Tp (peak temperature) of gelatinization ranged from 59.6 to 70.7°C among 14 tested lines (Table S1), appearing to be higher than the optimal temperature of their β-amylase activity in most test lines. It is likely that starch in the medium maturing lines with a high gelatinization temperature (approximately 70°C) could not be saccharified under the conditions where β-amylase was considerably denatured and lost its activity, and could therefore minimally convert starch into maltose. In contrast, in the late maturing lines, including ‘Hyoukei-kuro 3’ with a low gelatinization temperature (approximately 60°C), starch could be converted to a substantial amount of maltose due to the earlier and longer period of starch conversion (i.e. saccharification) during heat processing. In this study, we also found that starch gelatinization temperatures (especially peak temperature: Tp) were significantly correlated with maltose production in edamame seeds (Table 5). Masuda (2003, 2004) suggested, using a few varieties of soybean, that varietal differences in maltose content observed after heat processing were due to differences in the starch gelatinization temperature. Nomura et al. (2014) also reported a similar effect of the gelatinization temperature on the maltose production in soybeans. We here verified that the starch gelatinization temperature is a key for determining varietal differences in maltose productivity. Namely, our results suggest that β-amylase activity does not directly affect the amount of maltose produced after heat processing in edamame seeds, but rather a major difference in the maltose amount was affected by the conversion efficiency of starch into maltose. However, the reactive properties of β-amylase for starch hydrolysis in soybean seed tissue need to be confirmed by further study because it is considered that the degree of substrate hydration and heat transference in soybean seeds were difference among soybean lines under heat processing.
Variations in starch properties affecting gelatinization temperatureIt is known that amylopectin structure greatly affects the gelatinization properties of starch granules, and therefore, is one of the most important factors for the taste and processing suitability in rice (Okamoto et al., 2002; Umemoto et al., 2008). It is also well known that the diversity of rice starch is caused by the varietal difference of several enzyme genes for starch synthesis (Nakamura, 2002). In edamame seeds, the proportion of amylopectin short chains in the fa fraction (DP 6–12) showed a negative correlation with the gelatinization temperature (Table 6). A similar correlation between the proportion of amylopectin short chains and the gelatinization temperature was reported in rice (Inouchi, 2010; Vandeputte et al., 2003) and sweet potatoes (Noda et al., 1998). Starch granules are composed of alternating crystalline and amorphous lamellae. The crystalline lamellae are composed of double helices formed by amylopectin side chains (approximately DP 12–16), and the amorphous lamellae have a branched structure (Bertoft, 2015). It is known that the longer the double helices of the side chains are, the higher the stability of the crystalline lamellae, and that highly crystalline starch exhibits a high gelatinization temperature. Katayama et al. (2002, 2003) reported that a new sweet potato, ‘Quick Sweet’, with a low gelatinization temperature and quick cooking time was characterized by an increased distribution of short-chain of amylopectin and a low crystallinity of starch granules. Our results indicated for the first time that the starch of edamame seeds possessing high maltose productivity after heat processing exhibited a lower crystallinity due to their increased proportion of short-chain amylopectin (DP 6–12) with a lower gelatinization temperature.
The distribution pattern of amylopectin branch chain-length demonstrated that the late maturing lines had more short chains (DP 6–12) and fewer intermediate chains (DP 13–36) than the medium and intermediate late maturing lines, whereas no significant difference was found in long chains (DP>37) (Fig. 3). The pattern of difference in amylopectin chain-length observed in edamame was not the same as that found in rice endosperm, where either of starch branching enzymes I, starch synthase I, starch synthase IIa, starch synthase IIIa, or isoamylase 1 was assumed to be defective (Nakamura, 2015). Whether a single factor or multiple factors involved in amylopectin biosynthesis is responsible for the observed changes in amylopectin among soybean lines remains to be clarified.
It was notable that significant negative correlations existed between amylose content and the starch gelatinization temperature Tp (r = −0.784) and Tc (r = −0.866) (Table 6). The results agreed with previous reports on cooking rice (Umekuni et al., 2003; Yoshio et al., 1995). In addition, the gelatinization temperature range (Tc–To) was negatively correlated with amylose content. On the contrary, a positive correlation of the gelatinization temperature range (Tc–To) was found in the fb2 fraction % in amylopectin. These results suggested that the amylose and fb2 fractions of the amylopectin chain length affected the gelatinization progress, whereas the fa and fb1 fractions directly affected the gelatinization temperatures, especially the peak temperature Tp.
Variability in starch properties under different ambient temperatures during the maturation periodPossible effects of ambient temperature during the growing periods for the related factor of maltose productivity were also suspected because the biosynthesis of storage products is largely affected by environmental factors, including temperature, during soybean seed maturation (Taira, 1992).
The peak period of starch synthesis in typical soybean seeds ranged from 40 to 45 days after flowering (Saio and Monma, 1993) and coincided with the optimum timing for harvest of edamame. In the present study, we demonstrated that maltose productivity in edamame seeds with several starch properties was distinguished among different maturing groups of 14 black soybean lines (Fig. 1; Table 1–4). Furthermore, both the maturation period and the ambient temperature during the maturation period exhibited significant correlations with the starch gelatinization temperature in edamame (Table 7). These results suggest that the ambient temperature likely affects the varietal characteristics of the starch properties associated with the gelatinization temperature during the maturation period. A similar relationship between the ambient temperature during the maturation period and the gelatinization temperature was also reported in rice starch (Asaoka et al., 1984; Okuda et al., 2009) and amylose content in rice grains (Resurreccion et al., 1977; Sano et al., 1985; Yamakawa et al., 2007). It was reported that the fine structure of amylopectin changed with ambient temperature during the maturation period together with gene expression patterns and enzyme activity levels associated with starch synthesis (Inouchi et al., 2000; Jiang et al., 2003; Umemoto et al., 1999; Yamakawa et al., 2007). Similar trends were also reported in sweet potatoes (Noda et al., 1997) and wheat (Matsuki et al., 2003), but not in rice. In our study using soybean lines with different maturation properties, there was an approximately 4°C difference in the mean ambient temperature between the medium and late maturing lines during the second half of the maturation period (31st-M) (Table 1). Therefore, it was considered that each starch property related to the gelatinization temperature was affected by the ambient temperature during maturation of soybean varieties to varying degrees.
Prediction of varietal difference in maltose productivityIn this study of edamame seeds, we found that the maltose productivity was greatly affected by the starch gelatinization temperature under the influence of the chain length distribution of amylopectin and amylose contents. These properties were strongly affected by the ambient temperature during the maturation period. However, it remains unclear whether the gelatinization temperature is a primary determinant of the maltose production in edamame seeds or if some other factors are involved, because some lines (IL1, IL3, and IL4) in the intermediate late maturing group exhibited maltose production ability as high as that of the late maturing group (Fig. 1). Further investigating factors affecting maltose productivity by multiple regression analysis, we demonstrated the positive effect of starch content on maltose production, which may be caused by complementary effects of gelatinization temperature and starch content. This result showed the substantive importance for varietal differences in maltose productivity among the earlier maturing soybean lines than in the late maturing line ‘Tanbaguro’ because the ambient temperature did not affect the starch content during the maturation period (Fig. 1; Table 7). Therefore, it is considered that edamame black soybean lines can be harvested earlier than the standard black soybean ‘Tanbaguro’ without impairing the maltose production ability by line selection and/or breeding for a higher starch content.
Amylose content demonstrated a higher correlation with the maltose production (r = 0.786) than with the total starch content (r = 0.541) (Table 5). β-amylase is an enzyme that removes maltose units linked by α-1,4-glucosidic bonds of starch from non-reducing ends (Totsuka and Fukazawa, 1998). β-amylase can remove the α-1,4-glucosidic bonds of amylose, but cannot hydrolyze the α-1,6 bonds at branch points in amylopectin. Amylose exhibits a higher β-amylolysis limit of the conversion into maltose than amylopectin because amylose is mainly linked by α-1,4-linkage (Suzuki et al., 1981; Yoshio et al., 1995). Therefore, our results suggest that amylose content can directly affect maltose production. It is also thought that amylose content progress the gelatinization of starch based on the observed high correlation with the gelatinization conclusion temperature (Tc) and temperature range (Tc–To) (Table 6). Therefore, these results suggest that the effects of amylose content for starch gelatinization were rather different from those of chain length distribution of amylopectin.
It has been reported that the difference in temperature range contributed to the degree of crystalline heterogeneity within starch granules (Collado and Corke, 1997; Klein et al., 2013; Mweta et al., 2008; Vanier et al., 2012). Furthermore, amylose content affected the swelling power of starch granules by water-absorption due to chain length distribution of amylopectin (Pinto et al., 2012; Vasanthan and Bhatty, 1996; Zuluaga et al., 2007). The results of the present study also suggested that the above effects controlled the gelatinization properties of starch in edamame seeds. However, a possible relation between the amylose content and the maltose production needs to be examined in further studies.
In conclusion, our results demonstrated that the starch gelatinization temperature with the chain-length distribution of amylopectin and amylose content predominantly affected the maltose production after heat processing in edamame seeds. These starch properties were sensitive to the ambient temperature during the maturation period, and thus it can be presumed that the late maturing soybean lines generally have high maltose productivity. Our results also suggest that not only the lower starch gelatinization temperature, but also the starch content, effectively contribute to the increased productivity of maltose after heat processing in relatively earlier maturing soybean lines than ‘Tanbaguro’. Our study confirmed that several landraces in the Tanba region, which are harvested earlier than ‘Tanbaguro’, possessed as high a maltose productivity as ‘Tanbaguro’ with a high starch content. Furthermore, it is expected that breeding procedures based on genetic variations in the chain-length distribution of amylopectin and amylose content in soybeans may increase maltose productivity following genome analysis for high maltose production type edamame in the future.
The authors are grateful to Professor C. Nakamura, Ryukoku University, for his reading of, and suggestions for, the manuscript.