2018 Volume 87 Issue 3 Pages 389-394
2018 Volume 87 Issue 3 Pages 389-394
Improving water use and nutrient efficiency can play a pivotal role in ensuring sustainable production of horticultural crops. This study aimed to investigate the optimum moisture level that best management practices need to ensure for high-quality garden mum production, and also determine the feasibility of using a soil moisture sensor-based automated fertigation system for water and nutrient management for high-quality garden mum production. We used 20 5TE (Decagon Devices, Pullman, WA, USA) sensors to monitor and control fertigation based either on the substrate volumetric water content (θ, v/v) at 0.25, 0.35, 0.45, and 0.55 m3·m−3, or conventional greenhouse management culture practices. At harvest, most vegetative growth parameters were not significantly different across the treatments, but the leaf relative water content of plants under the 0.25 m3·m−3 treatment was lower than that of plants under other treatments, indicating that the plants were drought stressed. Although flower diameters and peduncle lengths were similar across the treatments, the number of flowers of plants under the 0.25 and 0.35 m3·m−3 treatments were 25–37% less compared to that of plants under the 0.45 and 0.55 m3·m−3 treatments, and the conventional culture practice, which suggested decreased quality of garden mums under water stress. Water savings without a decrease in product quality by adopting the automated fertigation system with the threshold θ values of 0.45 and 0.55 m3·m−3 were 34.2% and 42.7% of the conventional cultural practice, respectively. The soil moisture sensor-based automated fertigation system can therefore save a considerable amount of water and fertilizer and ensure efficient water and nutrient management for practical production of high-quality garden mums.
Efficient water management is an essential requirement for sustainable horticulture, and as such, various automated irrigation systems, including drip irrigation, boom systems, ebb and flow, and flood floor systems, have been used in greenhouse production of container-grown plants (Nelson, 2003). However, most of these automated systems are commonly equipped with timers or are manually turned on or off according to the growers’ decision or changing environmental conditions (Burnett and van Iersel, 2008). An ideal automated irrigation system would monitor the changes in either the substrate or plant water status in real time, and apply water or nutrient solution in the amount necessary to meet the water requirements of a plant. Capacitance soil moisture sensors have been successfully used to monitor the moisture content in horticultural substrates, and to control irrigation to maintain a consistent substrate moisture level with little or no leaching (Burnett and van Iersel, 2008; Nemali and van Iersel, 2006). The use of automated irrigation systems with soil moisture sensors has been investigated for various plants, ranging from woody species such as Rhododendron spp. (Lea-Cox et al., 2008) and Hydrangea (van Iersel et al., 2009) to herbaceous species such as petunias (Kim et al., 2011).
Chrysanthemums, Chrysanthemum × morifolium Ramat., are widely grown in the northern hemisphere as garden flowers, potted plants, and ground-cover types, and are popularly used as cut flowers. The market value of garden mums as potted herbaceous perennials has increased, and these were sold at a wholesale price of $124 million in the United States of America in 2015 (USDA, 2016). Potted mums can be irrigated in various ways, such as by using microtubes, capillary mats, and ebb and flow systems (Dole and Wilkins, 2005), but these irrigation systems have been commonly managed with timers and/or by using the growers’ empirical judgment. Although garden mums are known to not grow well when the substrate remains extremely wet (Dole and Wilkins, 2005), little is known about the amount of irrigation or fertilization needed to maximize plant growth without nutrient leaching and runoff. Efficient water and nutrient management are increasingly being considered important in greenhouse production because excessive irrigation and fertilization can increase production costs, as well as have serious environmental impacts. By controlling irrigation using soil moisture sensors, experienced growers can save up to 83% of the water currently used in the regular irrigation practices in a commercial nursery setting (van Iersel et al., 2009).
This approach has been previously tested using tensiometers for chrysanthemum cultivation in greenhouses under various moisture regimes. It was found that soil moisture tensions maintained at −1.5 and −3.5 kPa were suitable for minimizing water use while maintaining high chrysanthemum productivity (Lieth and Burger, 1989). Kiehl et al. (1992) reported that chrysanthemums grown under conditions with wide soil moisture tension ranges, with the low- and high-tension set-points set at −2 and −7 kPa, respectively, tended to have higher dry weights, than those grown under constant tension conditions with tensions set at −3 or −5 kPa. Dielectric moisture probes such as time-domain-reflectometry sensors and frequency-domain-reflectometry sensors have been considered as the most suitable soil moisture sensors for automated irrigation systems in plant production, since they have several benefits like ease of maintenance, relative cost-effectiveness, and reliability compared with the other types of soil moisture sensors (i.e., tensiometers, gypsum blocks, or neutron probes) (Lea-Cox et al., 2013). Therefore, these dielectric soil-moisture sensor-based automated irrigation systems have been effectively used in the production of horticultural plants (Bayer et al., 2013; Burnett and van Iersel, 2008; Thompson et al., 2007).
Although there have been several previous studies to improve water management for chrysanthemum production, few studies have compared the products grown by a commercial grower and those grown by a commercial farm, the two production systems varying in the water management approaches used. This study aimed to (1) determine the optimal volumetric water-content (θ, v/v) range necessary for commercial production of high-quality garden mums, and (2) compare the water use of conventional fertigation practices.
The experiment was conducted in a plastic greenhouse of a commercial garden mum nursery in Geumsan, Chungnam, Korea, between 14 August and 15 September 2015. The total ground area of the greenhouse was 1000 m2 with a central height of 4 m. During the experiments, the average temperature in the greenhouse was 20.1 ± 5.2°C, relative humidity was 58.8 ± 24.3%, and daily light integral was 12.0 ± 4.4 mol·m−2·day−1 (all values in mean ± SD) (Fig. 1). A total of 80 (5 treatments × 4 blocks × 4 sub-replicates) 3-month-old Chrysanthemum × morifolium ‘New Gigi’ pots were grown in 4-L containers filled with a commercial soilless substrate (mostly coir dust and peatmoss, and 10% perlite) mixed with sand at a 1:1 ratio (v/v). Containers were spaced 25 cm apart to minimize light interception by plants. Experiments began on 14 August, when flower differentiation had taken place, and flower buds began to appear on 20 August.
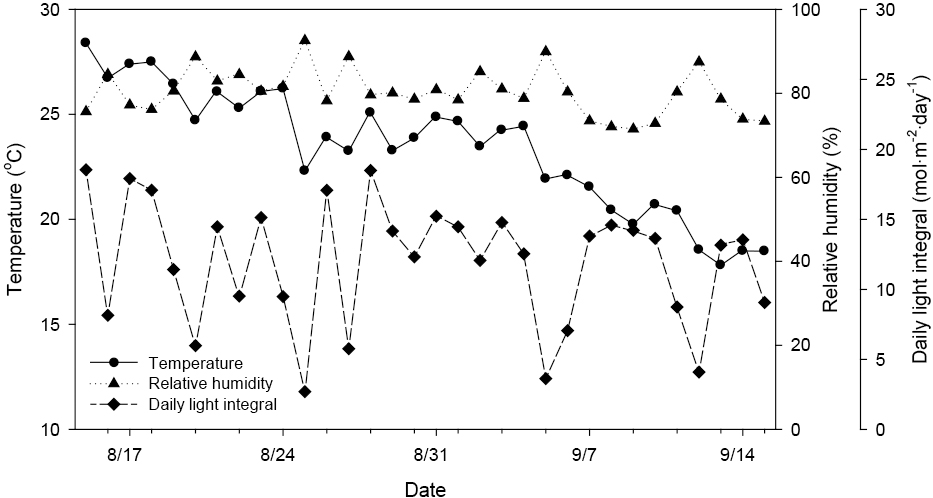
The daily average temperature, relative humidity, and daily light integral in the plastic greenhouse at a commercial garden mum farm during the experimental period.
In the farm where we conducted the experiments, the plants were drip fertigated 2–4 times per day depending on the weather condition and the plant growth stage. Drip fertigation was done with water-soluble fertilizer (20N-8.7P-16.6K, 20-20-20 Multi-feed; Haifa Chemicals, Haifa Bay, Israel) applied at a rate of 500 mg·L−1 for 1 min per cycle (approximately 33.3 mL per application per container) through a drip stake having a pressure compensated emitter (2 L·h−1; Netafim, Fresno, CA, USA). We set this as the conventional application (control) and installed an automated fertigation system on the farm separately. The automated fertigation system used in this study was modified from Nemali and van Iersel (2006). The θ (volumetric water content, v/v) of the containers was monitored and controlled by 20 soil moisture/EC sensors (5TE; Decagon Devices, Pullman, WA, USA). Each sensor was inserted to a depth of approximately 6.0 cm diagonally into the upper half of the substrate where the roots were distributed. All the 5TE sensors were connected to a datalogger (CR1000; Campbell Scientific, Logan, UT, USA) via SDI-12 protocols with 12 V excitation, and their dielectric values were converted to θ via substrate-specific calibration (θ = −0.0824 × V2 + 5.206 × V − 10.486, R2 = 0.98). When the θ reading of the soil moisture sensor in a container dropped below the set moisture level, a relay driver (SDM-CD16AC; Campbell Scientific) connected to the datalogger turned on the corresponding solenoid valve (S-390-2-R; Bermad, Kibbutz Evron, Israel) to fertigate. Until 20 August, all fertigation treatments maintained θ at 0.50 m3·m−3 to check the system and ensure uniform θs across all treatments before the experiment. After 20 August, the different θ points for the four different treatments were set at 0.55, 0.45, 0.35, and 0.25 m3·m−3, respectively. For comparison with the θ of conventional culture practice, four sensors were inserted into the containers under conventional fertigation. In conventional culture practice, the grower of the farm checked the state of the substrate frequently and irrigated based on his experience. The datalogger recorded the hourly average of θs of each experimental unit, which were comprised of four similar-sized plants, and the daily fertigation rates of each experimental unit for water use calculation.
Plant harvest and water use calculationAfter a month of treatment, two plants from each experimental unit were harvested, and their growth parameters, including plant height, plant width, shoot fresh/dry weight, flower number, flower diameter, peduncle length, chlorophyll content, and leaf relative water content, were measured. Chlorophyll contents were measured using a SPAD meter (Minolta, Osaka, Japan). Leaf relative water contents were measured by collecting the uppermost fully expanded leaf samples and by measuring the fresh weight of the leaves immediately after excision. Fresh weights of the fully turgid leaves were obtained after floating the samples in deionized water at 4°C for 6 h, and dry weights were determined after drying the samples at 60°C for 24 h. Leaf relative water contents were calculated as (fresh weight − dry weight)/(turgid weight − dry weight) × 100%. Water use was calculated from the number of irrigations as determined by the datalogger. Fertilizer cost savings per container were calculated as (saved fertigation volume (L) × nutrient solution application rate (mg·L−1) × fertilizer price per kg·10−6), assuming that 25 kg water soluble fertilizer cost $100, and the nutrient solution application rate was 500 mg·L−1.
Experimental design and statistical analysisA randomized complete block design with four blocks was used in the experiment. Plant growth parameters were analyzed using the general linear models procedure in SAS 9.3 (SAS Systems, Cary, NC, USA). Pairwise comparisons of group means were performed using Tukey’s honestly significant difference test with P = 0.05.
When plants were irrigated at the grower’s discretion in the farm, the θ fluctuated between 0.45 m3·m−3 and 0.55 m3·m−3 throughout the experimental period (Fig. 2). The substrate moisture level remained high, because the grower at the farm frequently checked the substrate moisture level, and kept the substrate wet. In the automated fertigation treatments, all irrigation treatments were maintained θ at 0.50 m3·m−3 until 20 August. After 20 August, θ in each of the four treatments was set at 0.55, 0.45, 0.35, and 0.25 m3·m−3, respectively, showing appropriate functioning of the automated system with very little variation within the same treatment between 1 September and 15 September (average of SDs 0.009, 0.008, 0.008, and 0.007 m3·m−3 for the 0.25, 0.35, 0.45, and 0.55 m3·m−3 treatments, respectively). However, the SD of θ in the conventional culture practice had an average of 0.011 m3·m−3, which was greater than that of the automated fertigation treatments. Previous studies have shown that an automated irrigation system with capacitance soil moisture sensors could maintain soil moisture within a narrow range during the cultivation of petunias (Kim et al., 2011). In our experiment, the θ in each treatment was also maintained above the set threshold level regardless of the set θ points except in the 0.45 and 0.55 m3·m−3 treatments due to technical errors in sensor reading, likely caused by damage to the sensors during the experiment. In actual, practical situations, sensor-based automation systems can malfunction because of several unexpected problems such as sensor damage, sensor disconnection, or power outage. Our results additionally support the use of multiple sensors and performance of careful maintenance to possibly secure safe production if one of the sensors develops a fault, and a fault-reporting system may be required for the automation system. Nevertheless, our results indicated that the soil moisture sensor-based automated irrigation/fertigation system could control irrigation/fertigation on a commercial scale.

Substrate volumetric water content (θ, v/v) changes during Chrysanthemum × morifolium ‘New Gigi’ cultivation using an automated fertigation system with various substrate water contents (θ = 0.25, 0.35, 0.45, and 0.55 m3·m−3) and conventional culture practice. The θ thresholds are shown as dashed horizontal lines. Data points and error bars indicate mean and standard errors (n = 4).
Drought-induced water stress or excessive water reduces the growth, development, and market quality of plants (Burnett and van Iersel, 2008). The leaf relative water content, as a numerical measure of water status, is commonly used as an indicator of water stress in plants. Plants grown at a θ threshold of 0.25 m3·m−3 had significantly lower leaf relative water content than those grown at higher θ thresholds (Table 1). van Iersel et al. (2010) reported that lower θ thresholds decreased the leaf relative water content, as well as the growth of petunia plants. In their study, the shoot dry weight of petunias decreased with a decrease in the θ thresholds during the course of the 3-week long experiment. The vegetative growth of other ornamental plants was found to decrease with decreasing θ thresholds (Bayer et al., 2013; Nemali and van Iersel, 2006). In our experiment, no significant differences were found in the plant height, chlorophyll content, flower diameter, shoot dry weight, or peduncle length among the plants grown at different θ thresholds (Table 1), probably because the treatment period formed only a part of the total vegetative growth cycle, whereas the plant width, shoot fresh weight, and flower number were affected by θ thresholds. Different θ treatments affected the flowering of the plants. The number of flowers decreased in the garden mum plants at θ of 0.35 m3·m−3 or less, indicating a decrease in product quality because of fewer flowers. A similar relationship between flowering and θ thresholds was reported by Cai et al. (2014); the flower number of roses increased with an increase in the θ threshold (from 0.1 to 0.3 m3·m−3), whereas no changes in the flower numbers were noted for θ thresholds between 0.3 and 0.4 m3·m−3. In our study, the θ thresholds at 0.45 m3·m−3 and 0.55 m3·m−3 were considered suitable for the cultivation of ‘New Gigi’ garden mum.

Plant height, plant width, chlorophyll content, shoot fresh weight, shoot dry weight, leaf relative water content, number of flowers per plant, flower diameter, and peduncle length of Chrysanthemum × morifolium ‘New Gigi’ under various substrate water contents and the conventional fertigation practice (Con.) at harvest (n = 8).
Under the conventional culture practice, the total volume of water-soluble fertilizer applied was 27.2 L per container during the experimental period; however, when θ was maintained at 0.25, 0.35, 0.45, and 0.55 m3·m−3, the volumes of water-soluble fertilizer used were only 2.2, 5.5, 8.2, and 9.5 L per container, respectively (Table 2). The total volumes of water used in the treatments were only 8.1–34.9% of the volume of water used in the conventional culture practice. Since the cost of water differs across countries and regions, the savings from such water conservation cannot be clearly estimated; however, approximately 70% of the water conservation from the current study would financially benefit the growers. If the water-soluble fertilizer costs $100 per 25 kg bag and 500 mg·L−1 is the general fertigation rate, then the estimated savings from the reduced fertilizer use is between $0.035 and $0.050 (Table 2), and the financial benefits may increase if the growing season is prolonged. Furthermore, the automated fertigation system promises to be less labor intensive, and by reducing leaching, also less polluting. Similarly, previous research with tree production reported that a soil moisture sensor-based automated irrigation system could provide benefits not only by saving water, but also by easing daily irrigation management by reducing pumping requirements and labor (Belayneh et al., 2013).

The total volume of nutrient solution applied and savings of water and fertilizer used for Chrysanthemum × morifolium ‘New Gigi’ cultivation using an automated fertigation system with various substrate water contents (0.25, 0.35, 0.45, and 0.55 m3·m−3) and conventional culture practice during a 33-day growing period.
Although lowering θ reduced the use of the nutrient solution, the 0.25 and 0.35 m3·m−3 threshold treatments produced unmarketable plants in our experiment, mostly because of quality loss owing to the production of fewer flowers (Table 1; Fig. 3). Lower θ may have delayed flowering of the plants by triggering a drought-induced decrease in plant cell expansion; however, this could not be confirmed due to harvest at the same date. If a lower θ can delay flowering without decreasing the quality of the plants, it would be beneficial to control the marketing dates. However, current results indicated that plants grown at θ of 0.25 m3·m−3 had lower leaf relative water contents than the plants with fewer flowers in the other treatments, which indicates a possible yield of poor quality products when θ is low, even if marketing is delayed. Therefore, we recommend that θ should be maintained within 0.45 and 0.55 m3·m−3 for the Chrysanthemum ‘New Gigi’ to reduce water and fertilizer use while ensuring the production of quality crops. In a previous study on hydrangeas, the irrigation amount at only 17.5% of that used in conventional practice was maintained using simple soil moisture sensor-controlled irrigation (van Iersel et al., 2009). The difference in water saving may be attributed to either the species or grower’s conventional irrigation practice. In our garden mum experiment, even if θ was maintained at 0.55 m3·m−3, the water used was only 35% of the amount used in conventional culture practice, which could contribute to markedly reducing the production costs of a garden mum nursery. Since most of the production practice for ornamental plants use not only water, but also water-soluble fertilizers, this may also reduce the budget for water-soluble fertilizers. In the future, efficient nutrient management by using an appropriate θ level should be adapted to maintain sufficient nutrient levels with reduced leaching.

Chrysanthemum × morifolium ‘New Gigi’ grown using an automated fertigation system with various substrate water contents (0.25, 0.35, 0.45, and 0.55 m3·m−3) and conventional culture practice at harvest. Bar = 10 cm.