2018 Volume 87 Issue 4 Pages 452-461
2018 Volume 87 Issue 4 Pages 452-461
‘Kurenainoyume’ is a newly released type 2 red-fleshed apple with pinkish flesh color. In our previous study, we revealed the effects of light condition on red coloration of the skin, flesh, and core of ‘Kurenainoyume’ apple fruit. In this study, we investigated the effects of temperature, shading, defoliation, and crop load on red coloration of the flesh. Low temperature improved flesh coloration; apples subjected to cooling treatment at 5°C or 10°C showed higher a* values of the Lab color space than the control (without cooling treatment), in a temperature-dependent manner. Low temperature reduced the soluble solid content to a lesser extent in some years, whereas fresh weight, malic acid content, and flesh firmness were not affected. Thus, the importance of temperature for flesh coloration was confirmed, similar to its effect on skin coloration. When the trees were covered with cheesecloth with a 50% shading rate from August 1 (82 days after full bloom: DAFB) to October 28 (170 DAFB), flesh coloration was negatively affected, and fresh weight, soluble solid, and malic acid contents were reduced relative to the control treatment (without shading). We also investigated the effects of defoliation (one-seventh of the control) and crop load (two- or threefold the crop load of the normal case control) on flesh coloration. Both defoliation and over crop load also decreased a* values, but their effects on the parameters related to fruit quality were not clear. Because all the treatments of shading, defoliation, and crop load relate to the efficiency of photosynthesis and the distribution of photosynthetic assimilates, our results also demonstrated that photosynthetic assimilates (carbohydrates) were one of the crucial factors affecting flesh coloration.
Interest in red-fleshed apple varieties is increasing in recent years and several kinds of red-fleshed apples have been released in Japan. For example, ‘Rose Pearl’ and ‘Ruby Sweet’ were released by National Agriculture and Food Research Organization (Abe et al., 2017; Okada, 2014). In addition to such red-fleshed type (type 2), another type (type 1) of red-fleshed apples has been also reported, in which type 1 apples show red coloration not only in the whole fruit including skin, flesh, and core but also in the leaves (Chagné et al., 2013; Würdig et al., 2014). In Japan, ‘Honey Rouge’ and ‘Columnar Rouge’ were released as type 1 apples by Shinshu University (Banno, 2016). Hence, molecular mechanisms underlying red-fleshed coloration in the two type apples have been reported; MdMYB10R6 of type 1 apples located in linkage group 9 (Espley et al., 2009), while type 2 apples develop red coloration only in the fruit flesh, which is controlled by MdMYB110a in linkage group 17 (Chagné et al., 2013; Umemura et al., 2013). Thus, in type 2 apples, the coloration of skin and fruit flesh is controlled by the different MdMYB10 (MdMYBA) or MdMYB110a genes (Umemura et al., 2013).
‘Kurenainoyume’ is a new type 2 red-fleshed apple cultivar, which was released in 2010 by Hirosaki University located in Aomori Prefecture (Igarashi et al., 2011). The fruit is flat with a dark red skin and matures during late-October to early-November in Aomori Prefecture. Average fresh weight of the matured fruit is 350 g, with approximately 13°Brix and 0.9% acidity (Matsumoto et al., 2016). The prominent feature is its pinkish flesh color attributed to the anthocyanin content (a kind of polyphenol). Although polyphenols usually have a bitter taste, ‘Kurenainoyume’ has a sweet and mild tart taste without astringency, irrespective of table or processing usage (Matsumoto et al., 2016). Due to its flesh pigmentation and sweetness, it has received greater focus in applications for processing (e.g., for juice, jam, dry fruit, pie, and tart production) by several companies (Matsumoto, 2017) in addition to table usage. For example, a juice product of ‘Kurenainoyume’, with a cherry-pink color, has been well-received by Hong Kong consumers, resulting in 200 bottles of sales in 2015, despite its relatively high price (2500 yen/bottle, 720 mL). As a result, apple growers, especially in Aomori, have become interested in the cultivation of ‘Kurenainoyume’.
However, a cork spot like physiological disorder (CSPD) in the skin of ‘Kurenainoyume’ apples has become a serious problem since the 2000s (Matsumoto et al., 2018a). To resolve this physiological disorder, we have successfully developed a cultivation technique by pre-harvest fruit bagging treatment using a light-impermeable double-layered paper bag (2-layer bag) at least from mid-July to late-August (Matsumoto et al., 2018a). Thus, fruit bagging treatment is a prerequisite technique for production of ‘Kurenainoyume’ apples without CSPD. However, it is well known that the fruit bagging treatment affects light conditions and, in turn, the red coloration of the apple skin, but little is known about its effect on flesh coloration. Hence, we investigated the effects of pre-harvest fruit bagging treatment on the red coloration of ‘Kurenainoyume’ apple skin, core, and flesh (Matsumoto et al., 2018b). We observed that light affected the red coloration of ‘Kurenainoyume’ apple skin, but did not have a marked positive effect on the coloration of the flesh (Matsumoto et al., 2018b). In contrast, it is known that several factors including temperature, plant hormones, and sugar content, other than light conditions, are involved in anthocyanin biosynthesis (Roubelakis-Angelakis and Kliewer, 1986; Xie et al., 2012).
Faragher (1983) showed that low temperature at 16–24°C was effective for anthocyanin accumulation in apple skin because low temperature accelerates the activity of phenylalanine ammonia-lyase (PAL), which is a key enzyme for flavonoid synthesis. Temperature effects on fruit skin coloration were also reported in grapes. Yamane et al. (2006) showed that anthocyanin accumulation in the skins of grape fruit was significantly higher at 20°C than at 30°C after the temperature treatment because the abscisic acid content, a plant hormone related to anthocyanin accumulation, and transcript levels of anthocyanin biosynthetic enzyme genes and a myb-related gene, VvmybA1, were higher at 20°C than at 30°C.
Yildirim et al. (2016) also reported that increased red coloration of the skin is inversely correlated with the increase of the crop load in ‘Gala, Galaxy’ apple. Yamane and Shibayama (2006a) recommended the application of trunk girdling to improve the fruit skin red coloration in grapes, and reducing the crop load to concentrate the photosynthetic assimilates to each fruitlet as an important measure to improve the red coloration of the fruit. Honda et al. (2017) demonstrated the effects of bagging, temperature, and crop load on flesh coloration in type 2 red-fleshed apple ‘Pink Pearl’. However, except for the report by Honda et al. (2017), there are no studies that have focused on commercially grown type 2 red-fleshed apple fruit like ‘Kurenainoyume’ and examined the effects of temperature, shading, defoliation, and crop load on flesh coloration.
In the present study, we first examined the effects of the temperature on red coloration of the flesh. We developed a cooling device to maintain the temperature lower than the atmosphere around the fruit-setting position (Fig. 1). Second, the effects of the following three treatments, which could relate to carbohydrate amount through photosynthetic assimilates, were studied; i) shading net with a 50% shading rate, ii) chemical defoliation (to reproduce the situation of reduction in photosynthesis assimilates), and iii) crop load.

Image for the fruit cooling treatment applied to ‘Kurenainoyume’ apple fruit. A: The fruit was covered with a light-impermeable double-layered paper bag (2-layer bag), a 5-mm diameter silicone tube was coiled up three times around the bagged fruit, and 5°C (5°C-treatment) or 10°C (10°C-treatment) of cold water was circulated. Control fruit was just covered by a 2-layer bag without water circulation. B: Images of the fruits captured by thermography (E60; Flir systems, OR, USA). The images were taken at 11 AM, October 18, 2013. The red and blue colors indicate high or low temperature, respectively. C: Average temperature of the fruit surface from 11:00 AM to 4:00 PM during the treatment period.
The experiment was conducted using one 7-year-old tree derived by grafting ‘Kurenainoyume’ apples onto a Malus prunifolia rootstocks, trained to a leader type form (6.0 m × 4.0 m planting) and grown at an experimental farm of Hirosaki University, known as Fujisaki farm (40°39'25''N, 140°29'9''E). The experiment was conducted in 2013 and 2014. In both years, approximately 50 fruitlets on the tree were randomly covered with a light-impermeable double-layered paper bag (2-layer bag, 194 mm × 162 mm; Masudaya Co., Aomori, Japan), approximately 40 days after full bloom (DAFB) (end of July). All the fruitlets were covered with the paper bag continuously until the harvest days; November 3, 2013 (163 DAFB) or October 29, 2014 (171 DAFB).
The fruit cooling treatment was conducted using a cooling water circulation device (CCA-1111; EYELA, Tokyo, Japan) equipped with a 5-mm diameter silicone tube. The silicone tube was coiled up three times around the bagged fruit (Fig. 1A), and 5°C (5°C-treatment) or 10°C (10°C-treatment) of cold water was circulated from October 6 (138 DAFB) to November 3 (163 DAFB) in 2013 and September 10 (122 DAFB) to October 29 (171 DAFB) in 2014. In both years, cold water circulation was conducted from 10 AM to 3 PM and 8 AM to 5 PM, respectively, that is, 5 h and 9 h treatment·day−1, respectively. Control fruit were just covered by a 2-layer bag without water circulation. The temperatures (5 or 10°C) were decided by pre-experiments considering the minimum temperatures of the treated periods. Each treatment was conducted for 12–15 fruits. The inner and outer atmospheric temperatures of the paper bags were measured using two-channel card loggers (MR5320; Chino, Tokyo, Japan) and the average of four fruits was used as each treatment data. The cooling condition was also checked by thermography (E60; FLIR, OR, USA) on October 18, 2013 (147 DAFB) (Fig. 1B).
Red coloration of the skin, the flesh, and the core was evaluated as a* values of the Lab color space, as multiple samples were measured simultaneously. Higher a* values indicated the high red coloration of the sample (Dong et al., 1995). The a* values of the flesh of the fruit were measured at two points facing each other via the core on the equatorial area using a color difference meter (NF333; Nippon Denshoku, Tokyo, Japan), and the average values were used as one data set. The flesh of the fruit was divided into two portions, inner or outer flesh representing 2.0–3.5 cm or 0.1–1.5 cm from the skin surface, respectively (Fig. 2). After the skin was removed, the flesh firmness was measured at two points on the equatorial area of the fruit using a penetrometer with an 11.1-mm tip (FT327; Facchini srl, Alfonsine, Italy). The total soluble solid content of the juice was determined using a digital refractometer (N-1; Atago, Tokyo, Japan). The total titratable acidity was measured by titration with 0.1 N sodium hydroxide (NaOH) and calculated as malic acid equivalent.

A diagram of the transverse section of apple fruit showing the experimental sampling parts.
This experiment was conducted in 2014 using four trees of ‘Kurenainoyume’ apple planted in the same row as the tree used in Exp. 1. Two trees were covered with cheesecloth with a 50% shading rate (Diorussell Bluck; Dio Chemicals, Ltd., Tokyo, Japan) using a steel pipe framework, whereas the other two trees were not covered (control). The shading treatment was conducted from August 1 (82 DAFB) to October 28 (170 DAFB; harvest), and the conventional horticultural care was applied to all trees. Sixty fruits were randomly harvested form each treatment and fruit weight, length, width, and flesh firmness were measured. The soluble solid content, titratable acidity of the juice, and red coloration of the flesh were measured using 40 fruits. The measurement methods were the same as those used in Exp. 1.
Effects of chemical defoliation treatment on fruit quality and flesh coloration (Exp. 3)This experiment was conducted in 2014 using three 36-year-old (11 years after top-grafting onto ‘Jonathan’) trees with ‘Kurenainoyume’ apple grafted onto a M. prunifolia rootstocks, trained to a flat open center form (5.0 m × 3.5 m planting) with four primary scaffolds grown at Fujisaki farm. One primary scaffold was randomly selected as the treatment scaffold and the remaining three scaffolds were treated with water (control). Chemical defoliation was conducted by spraying “John Color Pro” (12.5% of Chinomethionate, 25.0% of Fenitrothion, Agro-Kanesho Co., Ltd., Tokyo, Japan) solution, diluted 500 times with water, 45 days before harvest (DBH) (September 15; 127 DAFB). The treatments were applied using a 500 L/1000 m2 of solution using a power sprayer according to the instruction manual. The treatments exclusively defoliated the fruit cluster leaves, but were not effective for the shoot leaves. The trees received annual routine horticultural care, and the fruit were harvested on October 29 (171 DAFB). The number of spar leaves was counted at 9 DBH for 30 clusters. Ninety fruits were randomly harvested from each treatment, and fruit weight, length, width, and flesh firmness were measured. The soluble solid content, titratable acidity of the juice, and red coloration of the flesh were measured using 30 fruits. The measurement methods were the same as those used in Exp. 1. The skin color was indexed into five categories depending on the blush areas observed (0, 1, 2, 3, and 4: none, <30%, 30–60%, 60–90%, and >90%, respectively) (n = 30).
Effects of crop load on fruit quality and flesh coloration (Exp. 4)This experiment was conducted in 2013 using three ‘Kurenainoyume’ apple trees planted in the same row of the trees used in Exp. 3. Among the four primary scaffolds, two primary scaffolds were treated by the normal thinning (control), and the other two primary scaffolds were left with two times (×2-treatment) or three times (×3-treatment) the fruit at the thinning time (June 26; 33 DAFB), respectively. The ×3-treatment fruit trees were thinned only at the lateral and axillary positions and not the apical parts. The ×2-treatment was thinned only 1/3 of the fruit at the apical parts, in addition to the fruit thinned in the ×3-treatment. The control treatment was thinned at 2/3 of the fruit at apical parts, in addition to the fruit thinned in the ×3-treatment. The leaf/fruit ratios of each treatment were approximately 60, 30, and 20, respectively. Thinning of fruit at lateral and axillary positions was conducted on June 8 (15 DAFB) for all treatments and thinning of fruit at apical parts was conducted on June 26 (33 DAFB).
Fruit harvesting was conducted at two-time periods; early (October 30; 159 DAFB) or late (November 7; 167 DAFB). Twenty fruits were randomly harvested from each primary scaffold during each harvest period (a total of 60 fruits per treatment) and fruit fresh weight, length, and width were measured. The flesh firmness, soluble solid content, titratable acidity of the juice, and red coloration of the flesh were measured using 20 fruits selected randomly. The measurement methods were the same as in Exp. 1.
Statistical analysisThe data were analyzed using the Tukey-Kramer’s honest significant difference (HSD) tests or t-tests using the JMP IN software (SAS Institute, NC, USA), and significant differences among the treatments were determined. Unless otherwise stated, differences were considered statistically significant at P < 0.05.
Circulation of cold water effectively decreased the temperature of the fruit. Although the treatment duration in 2013 (5 h·day−1, 28 days/season) was shorter than in 2014 (9 h·day−1, 49 days/season), the average temperatures during daytime with 5°C-treatment and 10°C-treatment were 3.1°C and 1.5°C lower than in the control treatment, measuring 13.6°C and 15.2°C, respectively (Fig. 1C). Similarly, in 2014, average temperatures with 5°C-treatment and 10°C-treatment decreased to 8.6°C and 12.4°C, respectively (Fig. 1C). The check method by thermography also indicated effective cooling by the treatments (Fig. 1B).
In 2013, low temperature treatment (5°C-treatment and 10°C-treatment) did not change the fruit fresh weight, soluble solid content, malic acid content, or flesh firmness. Similar tendencies were observed in 2014; however, 5°C and 10°C treatments decreased the soluble solid content, whereas the 5°C-treatment, albeit to a small extent, increased the flesh firmness compared with the control and 10°C treatments (Table 1). As such, we concluded that the treatments did not have considerable effects on the parameters related to fruit quality.

Effects of low temperature treatments on fruit quality of ‘Kurenainoyume’ apples.
In 2013, low temperature treatments (5°C-treatment and 10°C-treatment) with 5 h·day−1 treatment duration (corresponding to 28 days/season) induced the coloration of both the inner and outer flesh of the fruit, and the degrees were correlated with the temperature level, i.e., lower temperature caused a redder coloration (Fig. 3A, B). The coloration was superior in the outer flesh compared with the inner flesh (Fig. 3B). With the control and the 10°C-treatment, some fruit showed less red coloration with a higher appearance rate of such phenotypes compared with the 5°C-treatment. In contrast, the 5°C-treatment resulted in a stable red flesh coloration in all examined fruit (Fig. 3A). Therefore, it was observed that the coloration stability of the flesh was also affected by the treatment.

Effects of low temperature treatments on flesh coloration of ‘Kurenainoyume’ apple fruit (2013). Cold water was circulated from October 6 to November 3 and from 10 AM to 3 PM. A: Image showing fruit coloration at harvest (November 3). B: The a* values of the inside and outside of the flesh at harvest. Vertical bars represent standard errors (SE). Different letters within the graph of the same portion show significant differences by Tukey-Kramer’s HSD tests at a 5% level of significance (n = 15).
For 2014, low temperature treatments (5°C-treatment and 10°C-treatment) with 9 h·day−1 treatment duration (corresponding to 49 days/season) also induced coloration of the flesh in a similar tendency to that in 2013. It is worth noting that the red coloration was more apparent under low temperature treatments in 2014 than in 2013 (Fig. 4A, B). Irrespective of the flesh portions, low temperature treatments induced clear and stable flesh coloration (Fig. 4A, B). In contrast, the control treatment induced low levels of flesh coloration in both the inner and outer flesh (Fig. 4B), with some variations depending on the fruit (Fig. 4A). These two-year results demonstrated that low temperature was important for flesh coloration. Next, we investigated the effect of the amount of photosynthetic assimilates on flesh coloration and fruit quality through shading treatment.

Effects of low temperature treatments on flesh coloration of ‘Kurenainoyume’ apple fruit (2014). Cold water was circulated from September 10 to October 29 and from 8 AM to 5 PM. A: Image showing fruit coloration at harvest (October 29). B: The a* values of the inside and outside of the flesh at harvest. Vertical bars represent SE. Different letters within the graph of the same portion show significant differences by Tukey-Kramer’s HSD tests at a 5% level of significance (n = 12).
Considering that fresh weight of the fruit under shading treatment was reduced by approximately 10% compared to control fruit, the shading treatment with cheesecloth with a 50% shading rate might have severely reduced the assimilates, possibly mainly derived from photosynthesis (Table 2). Moreover, soluble solid content and malic acid content were also reduced by the shading treatment, although the flesh firmness was not affected (Table 2).

Effect of shading treatment on fruit quality of ‘Kurenainoyume’ apples at harvest (October 28, 2014).
More uniform flesh coloration with lighter color was observed in the fruit from the shading treatment compared with the control fruit. In contrast, control fruit showed some variations in flesh coloration, but the color was deeper than that the fruit from the shaded treatment (Fig. 5A). The a* values of both the inner and outer flesh were higher in the control treatment than in the shading treatment. The flesh coloration of both the control and shaded tree fruit was higher in the outer than the inner flesh (Fig. 5B). These results suggest that flesh coloration was affected by the amount of assimilates. However, we cannot conclude clearly because shading was accompanied by a light reduction condition, which is also one of the important factors for anthocyanin biosynthesis. Indeed, the skin showed less red coloration (data not shown), which could be attributed to lower light conditions in the shading treatment. Therefore, we next considered defoliation treatment to elucidate the effect of the photosynthetic assimilates separately from light conditions.
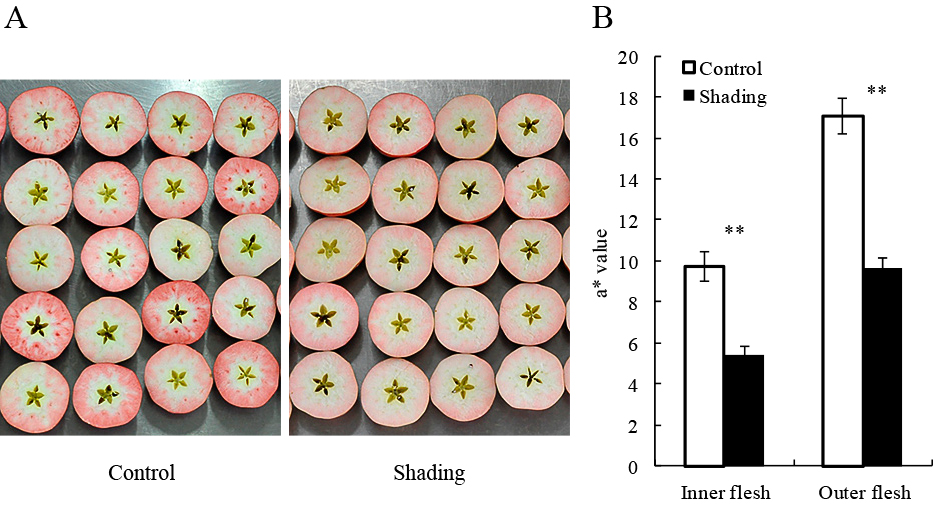
Effect of shading treatment on flesh coloration of ‘Kurenainoyume’ apple fruit (2014). A: Image showing fruit coloration at harvest (October 28). B: The a* values of the inside and outside of the flesh at harvest. Vertical bars represent SE. ** indicate significant differences between control and shaded fruit at P < 0.01, t-test (n = 20).
The chemical defoliation treatment effectively reduced the number of fruit cluster leaves and improved the color index of the skin. Moreover, this treatment did not affect the fresh weight, soluble solid content, malic acid content, or flesh firmness (Table 3). In contrast, the defoliation treatment reduced the development of flesh coloration compared with the control treatment (Fig. 6A); the a* values in both the inner and outer flesh of the defoliated fruit were significantly lower than those in the control fruit (Fig. 6B). To re-confirm the effects of photosynthetic assimilate amounts, we investigated the effect of crop load on flesh coloration and fruit quality.

Effects of chemical defoliation on fruit quality and number of fruit cluster leaves of ‘Kurenainoyume’ apples at harvest (October 29, 2004).

Effect of chemical defoliation treatment on flesh coloration of ‘Kurenainoyume’ apple fruit (2014). A: Image showing fruit coloration at harvest (October 29). B: The a* values of the inside and outside of the flesh at harvest. Vertical bars represent SE. ** indicate significant differences between control and defoliated fruit at P < 0.01, t-test (n = 30).
We harvested the fruits at two different harvest times, early (October 30; 159 DAFB) or late (November 7; 167 DAFB) harvest times, both of which are within the suitable harvest duration. When we harvested the fruit early, the ×3-treatment resulted in low fresh weight and low soluble solid content compared to the control treatment. Malic acid content of fruit of the ×2-treatment and the ×3-treatment was lower than that of the control treatment. There was no difference in the flesh firmness among the treatments (Table 4). During the late harvest time, fresh weight, soluble solid content, and malic acid content did not show any differences among the treatments. The flesh firmness of the fruit from the ×2-treatment showed a higher value compared with the control (Table 4). Compared to early harvest, late harvest showed a slight increase in fresh weight, soluble solid content, and malic acid content for both crop load treatments (Table 4). However, there were variations to some extent as observed in the control treatment; therefore, the effect of crop load on the parameters related to fruit quality was limited by the experimental duration, i.e., only one-year treatment.

Effects of crop load and timing of the harvest on fruit quality of ‘Kurenainoyume’ apples (2013).
In contrast, flesh coloration was influenced by crop load and harvest timing (Fig. 7). On October 30 (159 DAFB), flesh coloration of control fruit was superior to that of the ×2-treatment and ×3-treatment fruit (Fig. 7A, B). The a* values of both the inner and outer flesh of the control fruit were higher than those of the fruit of the ×2-treatment and the ×3-treatment (Fig. 7B). These differences were not observed at the late harvest time on November 7 (167 DAFB) (Fig. 7C, D). The flesh coloration progressed with late harvest, especially with the ×2-treatment and the ×3-treatment, compared to early harvest. The a* values of the outer flesh did not show differences among the treatments, although the a* values of the inner flesh in fruit of the ×2-treatment were slightly lower than those of the control fruit (Fig. 7D).

Effects of crop load and timing of the harvest on flesh coloration of ‘Kurenainoyume’ apple fruit (2013). A and C: Images showing fruit coloration on October 30 or November 7, respectively. B and D: The a* values of the inside and outside of the flesh at harvest on October 30 or November 7, respectively. Vertical bars represent SE. Different letters within the graph of the same portion show significant differences by Tukey-Kramer’s HSD tests at a 5% level of significance (n = 20).
Anthocyanins are induced by environmental factors such as solar radiation, cold temperature, and water stress, which can prepare plants for those stresses (Chalker-Scott, 1999). Anthocyanins also play an important role in seed dispersal by attracting wildlife to the fruit. Because anthocyanins in foods including fruit provide value for humans not only from the point of functionality, but also consumer preferences, intensive studies have been carried out to date (e.g., Silvestri et al., 2016). Accumulation of anthocyanins in fruit is affected by several factors, among which UV-B and low temperature (16°C) are important cues for its accumulation in apple fruit skin by inducing the expression of the anthocyanin biosynthetic genes such as chalcone synthase, anthocyanidin synthase, and UDP-glucose:flavonoid 3-O-glucosyltransferase (Dong et al., 1995; Ubi et al., 2006). In apples, the cold-induced bHLH gene, MdbHLH3, promoted fruit skin coloration (Xie et al., 2012). Moreover, when bagged fruit (dark-grown fruit) were exposed to sunlight, MdMYB1 (MdMYBA/10) transcript levels increased over several days, correlating with anthocyanin synthesis in red apple skin (Takos et al., 2006). In grapes, the associated MYB/bHLH/WD40 complex was decreased under low light intensity and dark conditions, which in turn resulted in a decrease in anthocyanin content through down-regulation of structural genes (Azuma et al., 2012). Red coloration of a type 2 ‘Kurenainoyume’ apple skin was drastically induced upon exposure to light conditions after paper bag removal (Matsumoto et al., 2018b). This result is in line with the previous reports that skin coloration is controlled by the MdMYB1/A/10 gene (Ban et al., 2007; Espley et al., 2007; Takos et al., 2006). Thus, apple skin coloration has been well studied at the molecular level. In contrast, detailed environmental cues for flesh coloration are not satisfactory, let alone their elucidation at the molecular level. However, the effects of sunlight on flesh coloration were not as large as expected (Matsumoto et al., 2018b). Therefore, in this study, we first investigated the effect of temperature on flesh coloration of ‘Kurenainoyume’ apple fruit.
Although our cooling apparatus could not reduce the temperature of the fruit to the set temperatures (5°C and 10°C), it could reduce the temperature by approximately 3.1–11.8°C lower than the control (Fig. 1C). As a result, it was obvious that flesh coloration of ‘Kurenainoyume’ apple fruit was improved in a temperature-dependent manner (Figs. 3 and 4). Thus, the importance of temperature for flesh coloration was obvious, similar to the case of skin coloration. Unfortunately, it is not clearly known if MdMYB110a is a light and/or low temperature responsive gene like MdMYB1. From our observations in the flesh coloration studies using ‘Kurenainoyume’ apple fruit, we assumed that MdMYB110a expression could be induced by light and low temperature, but it is more sensitive to low temperature than light. Elucidation of MdMYB110a expression under different light and temperature conditions by comparison with the case of MdMYB1 could be interesting and could open new avenues for our basic understanding of the mechanisms underlying anthocyanin accumulation in apples.
When the two-week duration treatments at 20°C and 30°C were carried out at four different developmental stages in a grape, ‘Aki Queen’ (Vitis labrusca L. × V. vinifera L., tetraploid), it was found that the level of anthocyanin content in the skin was higher at 20°C than at 30°C, and that the most sensitive stage for the temperature treatment was from one to three weeks after coloring began (stage III). The highest anthocyanin content was also observed in the grape skin treated at 20°C in stage III (Yamane and Shibayama, 2006b; Yamane et al., 2006). This result implies the presence of a sensitive stage to temperature depending on the fruit. In our study, there were some differences in the effectiveness of low temperature treatments between 2013 and 2014, and the treatment was more effective on flesh coloration in 2014 than in 2013 (Figs. 3 and 4). In ‘Kurenainoyume’ apple fruit, red flesh coloration starts from 120–140 DAFB, which corresponds to mid-September to early-October, with an average temperature of approximately 18–14°C (Japan Meteorological Agency, 2017). Our cooling apparatus started to operate from September 10 (122 DAFB) to October 29 (171 DAFB) in 2014, and from October 6 (138 DAFB) to November 3 (163 DAFB) in 2013; the treatment in 2013 may have been too late considering the suitable period for flesh coloration. In addition to the timing of the treatments, the duration of the cooling treatment in 2014 (9 h·day−1, 49 days/season) was longer than in 2013 (5 h·day−1, 28 days/season). Extension of the low temperature duration may also have contributed to improving flesh coloration. More in-depth studies are required to optimize the cooling period to achieve maximum red flesh coloration.
We found that low temperature was one of the important factors for flesh coloration in the type 2 red-fleshed apple ‘Kurenaninoyume’. We then investigated the effect of the amount of photosynthetic assimilates on flesh coloration and fruit quality. It is known that several factors other than light and temperature are also involved in anthocyanin biosynthesis (Roubelakis-Angelakis and Kliewer, 1986; Xie et al., 2012). In the ‘Aki Queen’ grape, trunk girdling showed a positive effect on fruit skin coloration (Yamane et al., 2008). In addition, trunk girdling combined with a low berry number and/or low crop load level, showed better results on fruit skin coloration compared with trunk girdling alone (Yamane and Shibayama, 2006b, 2007). These treatments, i.e., trunk girdling, low berry number, and low crop load, allow the berries to translocate a lot of assimilates, by which anthocyanin biosynthesis is accelerated because sugar is an important component of anthocyanins. In this study, to artificially reduce the assimilates, we used shading treatment and found a reduction in flesh coloration (Fig. 5), but we could not confirm if the results were due to low light conditions or to low photosynthesis ability (low assimilates). Therefore, we investigated the effects of defoliation and heavy crop load, both of which can affect the photosynthetic assimilates and their translocation into apple fruit on fruit coloration. The results suggested that both treatments reduced red flesh coloration (Figs. 6 and 7A). Honda et al. (2017) reported that excess fruiting resulted in decreased anthocyanin concentration in the flesh in type 2 apple ‘Pink Pearl’, re-confirming the importance of photosynthetic assimilates for flesh coloration. Remarkably, parameters related to fruit quality were not highly affected by defoliation (Table 3) and heavy crop load on late harvest (Table 4). These results may be due to the compensation effect or the use of the stored nutrients in the trunk and branches, but further studies including measurement of photosynthetic ability and kinetic analysis of assimilates are required to confirm our hypothesis.
In contrast, skin coloration was improved by defoliation (Table 3), but was not affected by heavy crop load (data not shown). Because the skin of ‘Kurenainoyume’ apples showed high sensitivity to light, faint light was thus sufficient for red coloration of the fruit skin (Matsumoto et al., 2018b), whereas early defoliation was not necessary, that is, even a shorter period of exposure to sunlight in fruit without defoliation leads to good skin coloration. In this study, we applied the defoliation treatment on September 15 (45 DBH; 127 DAFB), but considering the necessity for large amounts of photosynthetic assimilates for flesh coloration and high light sensitivity for fruit skin coloration, it may be better to apply the chemical defoliation treatment as late as possible.
In this study, we revealed that both low temperature and sufficient assimilates are necessary for red coloration of apple flesh. In practical growers’ orchards, it is nearly impossible to regulate ambient temperatures unless the cultivation is in a greenhouse. As an example, the effectiveness of a frost fan with a fine mist generator on apple skin coloration was evaluated at the Fukushima Agricultural Technology Centre during 2007–2010 using open fields (Hata et al., 2010). Skin coloration of ‘Fuji’ apple fruit tended to improve with this treatment; however, we think that this treatment is not realistic considering the cost of the apparatus. Therefore, northern cold areas and chilly areas at higher altitudes seem to be suitable for cultivation of the ‘Kurenainoyume’ apple. In addition, maintaining healthy leaves without injury by pests is a primary factor for achieving large quantities of assimilates. Another conceivable approach is application of carbon dioxide gas, but this is not suitable for open fields. Alternatively, low temperature treatments using cool water have been practically utilized in fruit such as the ‘Tsugaru’ apple after harvest to improve skin coloration. This shows the possibility that red flesh coloration in the type 2 red-fleshed apple ‘Kurenainoyume’ can also be improved after low temperature treatment after harvest. In addition, both flesh coloration and shipment could be controlled by cold storage. In Hirosaki city, there are already many facilities for cold storage of apples. Therefore, we intend to plan further experiments to elucidate the effects of cold storage of ‘Kurenainoyume’ apple fruit on flesh coloration.