2019 Volume 88 Issue 1 Pages 31-40
2019 Volume 88 Issue 1 Pages 31-40
In blueberry culture, when sulfur and NH4+ and K fertilizers are applied to soils, radiocesium in the soils may be released into the soil solution, absorbed by roots, and then translocated to fruit. We reanalyzed data from our previous experiment to evaluate soil factors affecting the concentration and total amount of natural stable Cs in blueberry organs and its translocation to fruit. During a 2-year pot experiment, 4-year-old rabbiteye blueberries (Vaccinium virgatum Aiton ‘Onslow’) were grown in three soils (Andosol, Cambisol, and Fluvisol) with or without soil treatment (acidification, NH4+ and K fertilization, or combined acidification-fertilization treatment). We measured the concentrations of 13 elements (N, Na, Mg, Al, P, K, Ca, Mn, Fe, Cu, Zn, Rb, and Cs) in samples of the soil solution and the blueberry fruit, leaves, branches and stems, and roots, as well as the pH of the soil solution. Acidification, fertilization, and combined treatment increased the Cs concentration in the soil solution within each soil. On the other hand, the Cs concentration in the whole bush was not changed significantly by any soil treatment. The Cs concentration in fruit, leaves, and branches and stems was significantly negatively correlated with concentrations of Na, Mg, K, and Ca in the soil solution. Among the three soils, the concentrations of these basic cations were lowest in the soil solution of the Cambisol. The fruit Cs concentration in the Cambisol did not change significantly with any soil treatments. In contrast, in the Andosol and Fluvisol, the fruit Cs concentration was significantly decreased by both acidification and fertilization. The whole-bush Cs content did not differ significantly among the soil treatments, whereas the percentages of Cs in fruit and roots depended greatly on the soil treatment within each soil, although the distribution trends relative to the control were opposite for fruit and roots. Our results suggested that the soil treatments to increase the concentrations of soil basic cations could reduce the rate of transfer of Cs to fruit and thereby contribute to a reduction in the Cs concentration in fruit, but not the whole-bush Cs content.
Blueberry (Vaccinium) species have shallow root systems, which exposes them to radiocesium accumulated in the surface soil. For instance, blueberry fruit in the Czech Republic contained high levels of 137Cs (15.66 to 86.54 Bq·kg−1), and the 137Cs concentrations were significantly correlated (r = 0.93) with soil 137Cs concentrations (184.32 to 520.54 Bq·kg−1) even 28 years after the 1986 Chernobyl Nuclear Power Plant accident (Cervinkova and Poschl, 2014). Similarly, blueberries imported to Serbia from Ukraine from the period August 2011 to December 2012 did not meet Serbia’s radiological safety criterion for 137Cs (150 Bq·kg−1), also apparently because of the nuclear accident at Chernobyl (Sarap et al., 2013). In Japan, following the Fukushima Daiichi Nuclear Power Plant accident in March 2011, levels of blueberry fruit radiocesium exceeded the maximum allowable limit for radiological safety (100 Bq·kg−1) in April–August 2012 (Ministry of Agriculture, Forestry and Fisheries, 2012), 2013 (Inao et al., 2014), and 2013 (Sugiura and Sakai, 2015). High levels of radiocesium in blueberry fruit may represent a long-term human health risk. Thus, suitable soil management to decrease radiocesium levels in blueberry fruit is important to reduce that risk.
Blueberry bushes grow well in acidic soils with a pH of 4 to 5 (Hart et al., 2006). One way to decrease soil pH for blueberry culture is to apply sulfur (Hart et al., 2006), which is oxidized by soil microorganisms to produce sulfate (Wainwright, 1984). The application of ammonium (NH4+) and potassium (K) fertilizers also improves plant growth; NH4+-N fertilizer produces better net photosynthesis and dry matter production (Claussen and Lenz, 1999; Tamada, 1993) than nitrate (NO3−)-N fertilizer. In a pot experiment, we grew blueberry bushes for 2 years in three types of soils treated by acidification with sulfur or combined NH4+ and K fertilization, or both acidification and fertilization, and found that these soil treatments caused natural stable Cs in the soils to be released into the soil solution (Matsuoka et al., 2018). However, the average stable Cs concentration in the soil solution during the experiment was not significantly correlated with the Cs concentration or its content in the blueberry bushes.
An increased supply of K to the soil has been reported to reduce plant uptake of radioactive or stable Cs (e.g., Jones et al., 1991; Shaw, 1993; Shaw and Bell, 1991). For instance, in winter wheat, Cs uptake was reduced by supplying NH4+ and K, and the K+ ion caused a greater reduction in Cs uptake than NH4+ (Shaw and Bell, 1991). However, few studies have investigated soil factors that reduce the uptake of radiocesium from soil by blueberry plants. Mandro et al. (2014) reported that K fertilizer combined with wood ash decreased the uptake of 137Cs by blueberries, and Iwabuchi (2014) reported that a high soil-exchangeable K content tended to reduce the concentration of radiocesium in blueberry fruit grown in Fukushima, Japan. A high level of radiocesium in blueberries may, therefore, be caused by a deficiency in soil K concentration. In addition, low soil pH and a low soil Ca concentration may lead to high radiocesium concentrations in wild blueberry fruit in Mt. Azuma, Japan (Sugiura and Sakai, 2015). These findings strongly suggest that the uptake of radioactive or stable Cs by blueberry bushes and its translocation to fruit are not determined solely by the Cs concentration in the soil solution. However, we have limited information about how the concentration and content of radioactive or stable Cs and its translocation to blueberry bush organs is affected by soil properties, and in particular by the cation composition of the soil solution.
In this study, we reanalyzed the data from our previous experiment (Matsuoka et al., 2018) and evaluated how the original soil properties and soil acidification and fertilization treatments affected the concentration and content of natural stable Cs in blueberry organs and its translocation to the fruit.
A 2-year pot experiment was carried out with three types of soils, an Andosol, a Cambisol, and a Fluvisol (FAO et al., 1998), and four soil treatments, control (no treatment), acidification, combined NH4+ and K fertilization, and combined acidification-fertilization treatment, for a total of 12 soil type-soil treatment combinations. Each pot was planted with a single 4-year-old rabbiteye blueberry bush (Vaccinium virgatum Aiton ‘Onslow’), and each treatment was conducted in triplicate (for a total of 36 pots). Details of the experimental procedures and of the sampling and analysis of the soil solutions in the root zone and blueberry bushes were given by Matsuoka et al. (2018).
In brief, soils were collected from the top 20 cm of experimental fields at Tsukuba, Kasumigaura, and Takatsuki, Japan. The Andosol (Tsukuba) had the highest total C content (40 g·kg−1), and the Cambisol (Kasumigaura) had the lowest total C content (24 g·kg−1). The Cambisol had a lower pH (5.7) than the Andosol (pH 6.4) or the Fluvisol (Takatsuki; pH 6.7). The Fluvisol contained higher amounts of water-soluble NO3−, Al, P, Fe, Cu, Zn, and Cs than the other two soils (Matsuoka et al., 2018). All soils were air-dried and passed through a 2-mm sieve before being used in the pot experiment.
For acidification treatment, 3 kg of soil was thoroughly mixed with sulfur powder at 5 g·kg−1 to adjust the soil pH to between 3 and 4. For fertilization treatment, 3 kg of soil was thoroughly mixed with 2.29 g ammonium chloride (NH4Cl) and 1.14 g potassium chloride (KCl) at application rates of 0.2 g N·kg−1 and 0.2 g·K kg−1, respectively. The soil mixture was kept moist by adding tap water, and then part of the soil mixture was placed in a 7-L plastic pot with an open bottom (210 mm high, 240 mm in diameter). Next, two porous-cup soil solution samplers (DIK-8393; Daiki Rika Kogyo, Saitama, Japan) were installed in each pot near the center position at 5–6 cm above the bottom and then covered with the soil mixture (for details see Matsuoka et al., 2018) for later nondestructive collection of soil solution samples by suction. Finally, a single blueberry bush was planted in each pot by holding the bush inside the pot and then filling the pot with the rest of the soil mixture.
The pot experiment was conducted in a glass room in Tsukuba, Japan, from 18 March 2014 to 17 September 2015. The date on which the blueberry bushes were planted was considered day 0 of the experiment. The average temperature in the glass room from 30 October 2014 to 28 September 2015 was 16.6°C. The temperature was not controlled during the whole experimental period. On 30 March 2015, in the second year of the experiment, the fertilized pots received an additional fertilization at the same application rates as in the first year by adding the dissolved nutrient solution to the surface of the pot. The pots were supplied with tap water through an automatic irrigation system during the experiment, with the amount of water calculated to minimize drainage loss at the bottom of the pots. The blueberries were cross-pollinated with another variety of rabbiteye blueberry (‘Tifblue’) with the aid of honeybees, and they were not pruned to avoid any loss of Cs in the excised material.
Sampling and analysis of the root-zone soil solutionThe soil solution in the root zone was collected eight times during the whole experimental period: on 29 March (day 11), 28 April (day 41, at flowering), 29 June (day 103, at harvest), and 1 November (day 228, after harvesting) in 2014 and on 23 March (day 370), 26 April (day 404, at flowering), 28 June (day 467, before harvesting), and 30 August (day 530, at harvest) in 2015. On the day before each sampling, to control for the effect of soil moisture content on the element concentrations in the soil solution, the soil water content in the pots was adjusted to 60% of the maximum water-holding capacity by adding tap water. Within 24 h of the watering, soil solution (< 20 mL) was collected from each pot, and the soil solution samples were frozen until analysis. Each soil solution sample was analyzed by inductively coupled plasma mass spectrometry (ICP-MS, Agilent 7700 Series; Agilent Technologies, Tokyo, Japan) to determine the concentrations of 12 elements (Na, Mg, Al, P, K, Ca, Mn, Fe, Cu, Zn, Rb, and Cs); for N, NH4+-N, the concentration was determined by the indophenol method (Keeney and Nelson, 1982), and the NO3−-N concentration was determined by the method of Cataldo et al. (1975). The pH of each soil solution sample was also measured with a glass electrode pH meter.
Sampling and analysis of blueberry fruit, leaves, branches and stems, and rootsThe blueberry fruit was sampled during the harvesting season between 23 June and 30 October in 2014 and between 29 June and 17 September in 2015. The fruit was collected once each week. The fruit samples were frozen and then freeze-dried by using freeze dryers (FD-2MM and FDc-2BU; Nihon Techno Service, Ibaraki, Japan; FDU-2110; EYELA, Tokyo, Japan). The dried fruit samples were weighed, ground into powder, and stored in desiccators until analysis. After the second fruit harvest on 17 September 2015, each blueberry bush was divided into the leaves, branches and stems, and roots. The leaf samples were dried in an oven at 70°C until they reached a constant weight. The root samples were washed in tap water. The samples of branches and stems, and roots were cut into 1-cm pieces, and oven-dried at 70°C until reaching a constant weight. The oven-dry weights were measured, and then the samples were ground into powder and stored under ambient conditions until analysis. The plant samples were digested in concentrated HNO3 (60.0–62.0%), and the concentrations of the 12 elements (Na, Mg, Al, P, K, Ca, Mn, Fe, Cu, Zn, Rb, and Cs) in the digested solutions were measured by ICP-MS. The concentration of N was also measured by the dry combustion method using an NC analyzer (Sumigraph NC-220F; Sumika Chemical Analysis Service, Osaka, Japan). The Cs content of each organ was calculated by multiplying the measured Cs concentration by the dry weight of that organ. The whole-bush content was obtained by summing the contents of the organs.
Statistical analysisWe compared the Cs concentration and content in each organ and the whole bush between the control (no treatment) and the other soil treatments (acidification, fertilization, and combined acidification-fertilization treatment) within each soil type by one-way ANOVA. We also determined the effects of soil type, soil treatment, and soil type × treatment interaction on the Cs concentration and content in each organ and the whole bush by two-way ANOVA. We further evaluated Cs transfer to the fruit and other organs and factors influencing the transfer, and performed Pearson’s correlation analyses (r) in which we compared (1) Cs concentrations among the blueberry organs, (2) Cs concentrations in the organs and the whole bush or Cs contents in the whole bush with the average concentrations of the 13 elements and the average pH in the soil solution, and (3) concentrations of Cs in the organs and whole bush with the corresponding concentrations of the other 12 elements. All statistical analyses were performed with Microsoft Excel 2010 and Excel TOUKEI 2012 software (Social Survey Research Information, Tokyo, Japan).
Our previous paper reported average Cs concentrations in the root-zone soil solution during the experimental period in each of three soil types and four soil treatments (Table 1, Matsuoka et al., 2018). The Cs concentration in soil solution was significantly affected by soil type, soil treatment, and soil type × treatment interaction. In addition, in each soil, the Cs concentration in the soil solution was most increased compared to the control (no treatment) by combined acidification-fertilization treatment. In the Andosol and Cambisol, the Cs concentration in the soil solution was in the order fertilization > acidification > control (no treatment), and in the Fluvisol, it was acidification > fertilization > control (no treatment). The average Cs concentration in the soil solution was highest in the Cambisol and lowest in the Fluvisol in acidification, fertilization, and combined acidification-fertilization treatment.

Average Cs concentration in the root-zone soil solution during the whole experimental period for the three soil types and four soil treatments.z
When we compared Cs concentrations in the blueberry organs and the whole bush among the three soil types and four soil treatments (Table 2), we found that in the first year, the Cs concentration in the fruit did not differ significantly among soil types or in soil type × treatment interaction, but it differed significantly among soil treatments. In the second year, the Cs concentration in the fruit was significantly affected by soil type, soil treatment, and soil type × treatment interaction. In the Andosol and Fluvisol, the fruit Cs concentration when compared with the control (no treatment) was reduced significantly by acidification, fertilization, and combined acidification-fertilization treatment; that is, in these two soils, no treatment resulted in the highest fruit Cs concentrations. In the Cambisol, however, though the fruit Cs concentration compared with the control (no treatment) was reduced greatly (though not significantly) by fertilization, it was increased (though not significantly) by acidification. The Cs concentration trends in the branches and stems were similar to those in the fruit. In the leaves and roots, the Cs concentration did not differ significantly among soil treatments within a given soil type. However, the leaf Cs concentration differed significantly among soil types, and the root Cs concentration differed significantly among soil types and soil treatments. The Cs concentrations were higher in the roots than in any of the other organs, reflecting the Cs accumulation in the roots. Overall, the whole-bush Cs concentration did not differ significantly among soil treatments within a soil type, but it was significantly affected by soil type, although not by soil treatment or soil type × treatment interaction.

Cs concentration in each blueberry organ and in the whole bush in the three soil types and four soil treatments.
Correlation analysis of the Cs concentrations among the blueberry organs (Table 3) showed that the Cs concentrations in the aboveground organs (fruit, leaves, and branches and stems) were significantly positively correlated; in particular, the fruit Cs concentration was strongly correlated with the branch and stem Cs concentration (r = 0.959, P < 0.01) and less strongly correlated with the leaf Cs concentration (r = 0.651, P < 0.05). The root Cs concentration was not significantly correlated with the Cs concentration in any of the aboveground organs.

Pearson’s correlation coefficients (r) between Cs concentrations in blueberry organs.
Correlation analysis of the Cs concentration in each blueberry organ and in the whole bush with the average concentration of each of the 13 elements and the pH in the root-zone soil solution is shown in Table 4. The Cs concentrations in the fruit, leaves, and branches and stems were significantly negatively correlated with the concentrations of Na, Mg, K, and Ca in the soil solution. The Cs concentration in the roots, however, was significantly negatively correlated only with the soil solution NO3− concentration and pH, and it was significantly positively correlated with the soil solution Al concentration. Similar to the roots, the whole-bush Cs concentration was significantly negatively correlated with soil solution NO3− concentration and significantly positively correlated with the soil solution Al concentration.
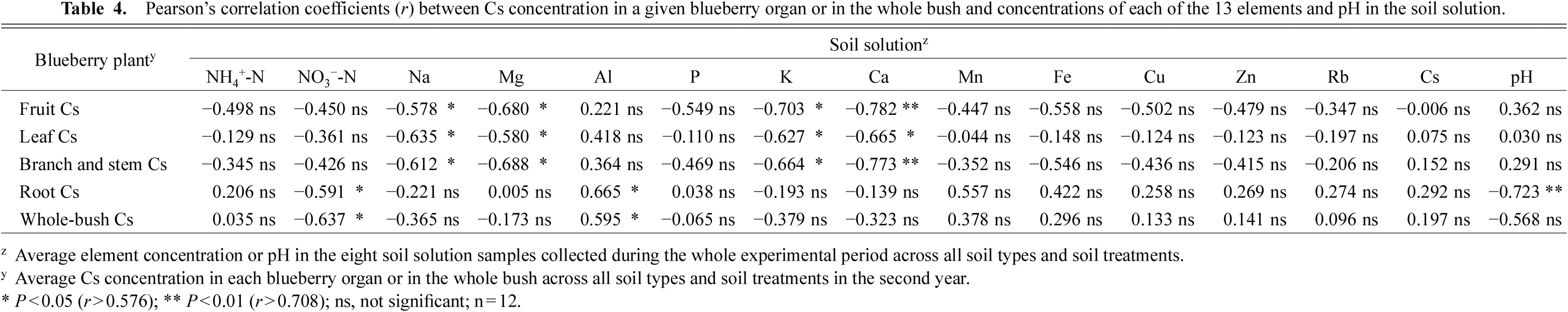
Pearson’s correlation coefficients (r) between Cs concentration in a given blueberry organ or in the whole bush and concentrations of each of the 13 elements and pH in the soil solution.
Correlation analysis of the Cs concentration in each blueberry organ and in the whole bush with the corresponding concentrations of the other 12 elements (Table 5) showed that the Cs concentration in the fruit was significantly negatively correlated with N, P, and K concentrations in the fruit, but significantly positively correlated with the fruit Ca concentration. The root Cs concentration, however, was significantly positively correlated with the corresponding Al, Mn, Fe, and Rb concentrations. In the whole bush, similar to in the roots, the Cs concentration was significantly positively correlated with the corresponding concentrations of Al, Fe, and Rb. The correlations were thus considerably different between the fruit and roots. These results indicate that the association of the Cs concentration with concentrations of other elements depended greatly on the specific blueberry organ examined.

Pearson’s correlation coefficients (r) between Cs concentration and those of the other 12 elements in each blueberry organ and in the whole bush.
Our comparison of the Cs contents in the blueberry organs and in the whole bush among the three soil types and four soil treatments (Table 6) showed that, in each bush, most of the Cs content was located in the roots, regardless of soil type or soil treatment. In fact, in all soil types and soil treatments, at least 44% of the total Cs content in the whole bush was located in the roots. Thus, the whole-bush Cs content was strongly affected by the root content, with the result that within a soil type, the whole-bush Cs content did not differ significantly among soil treatments, and there was no significant soil type × treatment interaction. In contrast, the Cs content in the fruit differed significantly among soil types, soil treatments, and the soil type × treatment interaction. In the Fluvisol, the fruit Cs content was significantly higher in the control (no treatment) than in the other soil treatments, whereas in the Cambisol, the fruit Cs content was significantly higher in acidification than in the control (no treatment). However, in the Andosol, the fruit Cs content did not differ significantly among soil treatments. The Cs content in the leaves was affected significantly by soil treatment, but not by soil type or soil type × treatment interaction. The Cs content in the branches and stems was significantly affected by soil type, but not by soil treatment, whereas the soil type × treatment interaction was significant.

Cs content of each blueberry organ per bush and of the whole bush, and the distribution of Cs in each organ as a percentage of total Cs in the whole bush for the three soil types and four soil treatments.
Acidification, fertilization, and combined acidification-fertilization treatment reduced the percentage of Cs in the fruit (though not significantly) from 20% (control) to 5–13% in the Andosol, but reduced it significantly from 25% (control) to 3–13% in the Fluvisol. Acidification, fertilization, and combined acidification-fertilization treatment significantly increased the percentage of Cs in the root from 49% (control) to 64–84% in the Andosol, and these three treatments significantly increased the Cs percentage in the roots from 44% (control) to 45–90% in the Fluvisol (Table 6). In contrast to the Andosol and Fluvisol, in the Cambisol, acidification, fertilization, and combined acidification-fertilization treatment increased the percentage of Cs in the fruit (but not significantly) from 5% (control) to 8%, whereas these three treatments reduced the percentage of Cs (but not significantly) in the roots from 87% (control) to 77–82%. The distribution trends of Cs in the leaves and the branches and stems were similar to distribution trends in the fruit in the Cambisol, but not to those in the fruit in the Andosol or Fluvisol. These results show that soil treatment strongly influenced the percentage distribution of Cs in the blueberry organs.
We further analyzed the relationships between the whole-bush Cs content and the average concentration of each of the 13 elements and pH in the root-zone soil solution (Table 7). The whole-bush Cs content was significantly negatively correlated with the NO3− concentration and the pH in the soil solution and significantly positively correlated with the Al concentration in the soil solution. These results are consistent those of the correlation analysis comparing the root Cs concentration with the average concentrations of the 13 elements and pH in the root-zone soil solution (Table 4), indicating that the whole-bush Cs content was affected strongly by the root Cs concentration.

Pearson’s correlation coefficients (r) between Cs content in the whole bush and the concentrations of 13 elements and pH in soil solution.
The Cs concentration in the root-zone soil solution was increased greatly by acidification, fertilization, and combined acidification-fertilization treatment within a given soil compared with the control (no treatment) (Table 1). This result is consistent with previous findings. At acid pH levels, less Cs is sorbed, and the effect of pH on Cs sorption shows a similar pattern in various soils (sandy loam, loam, clay loam, and clay) (Giannakopoulou et al., 2007). Furthermore, 137Cs is released from 137Cs-contaminated soil when NH4+ or K fertilizer solutions are added to the soil (Chiang et al., 2008). However, in our study, the Cs concentration in the whole blueberry bush was not changed significantly by any soil treatment within a given soil (Table 2). This result is in part attributable to the fact that the Cs requirement of the bushes was lower than the amount supplied via the soil solution. In the second year of our experiment, the fruit Cs concentrations in the Andosol and Fluvisol were reduced significantly by acidification, fertilization, and combined acidification-fertilization treatment (Table 2). In the Cambisol, however, the fruit Cs concentration was reduced greatly (but not significantly) by fertilization and increased (but not significantly) by acidification. This result suggests that the soil treatments strongly influenced the fruit Cs concentration, but the nature of the influence depended greatly on the soil type.
In our correlation analysis performed to identify the key cations in the soil solution affecting the Cs concentration in each blueberry organ and in the whole bush (Table 4), the Cs concentrations in the fruit, leaves, and branches and stems were significantly negatively correlated with the Na, Mg, K, and Ca concentrations in the soil solution, but they were not significantly correlated with the Cs concentration in the soil solution. This result confirms that the Cs concentration in the soil solution is not the main factor affecting the Cs concentration in the blueberry organs and the whole bush. Among the three types of soils, the concentrations of Na, Mg, K, and Ca in the soil solution of the Cambisol were the lowest, and the K concentration in the soil solution was significantly increased by fertilization, but not by acidification (Matsuoka et al., 2018); this increase in the K concentration in the Cambisol is probably why fertilization tended to reduce the fruit Cs concentration, whereas acidification tended to increase the fruit Cs concentration (Table 2). Unlike the Cs concentrations in the aboveground organs, but similar to the whole-bush Cs concentrations, the root Cs concentrations were not significantly correlated with the Na, Mg, K, and Ca concentrations in the soil solution (Table 4). These results suggest that increases in the concentrations of the basic cations, especially K, in the soil solution contribute to a reduction in the Cs concentration in the blueberry fruit, but not in the roots or the whole bush.
The translocation of Cs from blueberry root to fruitThe significant positive correlations among the Cs concentrations in the aboveground organs (fruit, leaves, and branches and stems) (Table 3) suggest that the strong Cs accumulation by the roots and the translocation of Cs from the roots to the aboveground organs resulted in an almost equal distribution of Cs among the aboveground organs. Previous studies have also shown that radiocesium concentrations in blueberry fruit and the current year’s branches increase together (Inao et al., 2014). In addition, Carini et al. (1999) reported that in grapevines and apple and pear trees, a transfer of 134Cs between the fruit, leaves and branches occurred within 50 days after wet deposition of the 134Cs on fruit or leaves; they also showed that less 134Cs was transferred to the fruit and leaves on non-contaminated branches than to those on contaminated branches. Their results suggest that, as in the Cs translocation in the blueberry bushes in our study, 134Cs is transferred among all aboveground organs in grapevines and apple and pear trees.
We further investigated how different soil treatments influenced the Cs uptake by the blueberry bush and its translocation from the roots to fruit. Because the whole-bush Cs content did not differ significantly among any soil treatments within a soil type (Table 6), we compared the distribution of the Cs content among the organs as a percentage of the total whole-bush Cs content. Within a given soil, the percentage of Cs in both the fruit and root depended greatly on the soil treatments, but the distribution trends in the fruit and root in each soil type were opposite. The effects of acidification, fertilization, and combined acidification-fertilization treatment on the Cs distribution depended greatly on the soil type; in the Andosol and Fluvisol, the soil treatments effectively reduced the percentage of Cs in the fruit, but in the Cambisol, they increased it. These results show that soil treatments strongly influenced the percentage distribution of Cs in the fruit, even though they did not influence the Cs content of the whole bush.
Several studies have reported decreased plant uptake of radiocesium at high levels of soil K and Ca. Mandro et al. (2014) reported a reduced uptake of soil 137Cs by blueberry bushes after the application of K, and Shaw (1993) reported that K+ and Ca2+ in the soil were effective ions at blocking radiocesium uptake by crop plants. However, we found that the application of a combined NH4+ and K fertilizer did not significantly change the Cs content in whole blueberry bushes. Unfortunately, there are no other studies on the effect of soil treatments on the distribution of Cs in blueberry bushes. Therefore, whether the application of Na, Mg, K, and Ca fertilizers can actually reduce the Cs concentration in the fruit requires further investigation.
In conclusion, our results confirm that the concentration and content of natural stable Cs in blueberry bushes and its translocation to the fruit are not determined solely by the Cs concentration in the soil solution. Moreover, the effects of soil treatments on the Cs concentration in the fruit and the percentage of Cs in the fruit depended greatly on the soil type. Further research is needed to evaluate whether these findings are also applicable to radiocesium, as well as to field conditions in an open system.
We thank Dr. T. Matsunaga, Central Region Agricultural Research Center of the National Agriculture and Food Research Organization (NARO) for technical support with the ICP-MS analysis; R. Hasegawa and N. Ishikawa, Institute of Fruit Tree and Tea Science, NARO, for assistance in preparing the pot experiment and with the plant and soil sampling and pretreatments; and Dr. T. Kawai, Kyoto University Farm (present affiliation, Okayama University) for allowing us to collect the Takatsuki soil.