2019 Volume 88 Issue 2 Pages 232-244
2019 Volume 88 Issue 2 Pages 232-244
An excess or lack of soil moisture are significant abiotic stresses that reduce the average yield for vegetable crops worldwide. The responses of ‘Natsunoshun’, a processing tomato cultivar, to water stress at three growth stages, first flower differentiation, first flowering, and fruit development, were investigated over a two-year period. The year effect on yield was not significant; however, the growth stage and the type of water stress at a particular growth stage affected yield significantly. Either an excess or lack of soil moisture after the first flowering stage were significant in reducing yield. The decrease was related to the average weight of the fruit rather than the number per plant. Under dry conditions, fruit number was the same as control plants, but there was a decrease in the reddish mature fruit ratio. On the other hand, under wet conditions plant biomass decreased, especially in the roots, even if there was a transition from wet to dry conditions. We conclude that excessive soil moisture during the first flowering stage produces slower CGR and decreased biomass in the roots, which we believe is the reason for the yield decline.
Domestic demand for tomatoes for processing is increasing annually because of a strong trend towards healthy foods. Tomato paste is a major ingredient in many popular products such as ketchup, sauce, puree and juice. Hokkaido, a major agricultural region in northern Japan, which has a 200% food self-sufficiency rate, only produces 3% of domestic processing tomatoes (MAFF, 2015). However, processing tomato production in Hokkaido has recently garnered more attention because climate change has become a serious worldwide problem and high temperatures are a major environmental factor limiting tomato productivity (Silva et al., 2017), even in Japan (Sato, 2006). The reproductive stage appears to be especially vulnerable to temperature increases (Hedhly et al., 2009).
However, one of the severe problems for Hokkaido agriculture is the decrease in the number of farm households, especially in paddy field areas where the farm population is drastically decreasing as farmers leave the agriculture sector or retire (aging population problem). Basically, in Hokkaido, the cultivation area per farmer is more than 10 times greater than the national average (MAFF, 2017), so a low-cost production system requiring minimum labor is extremely desirable. To realize this goal, the NARO Bio-oriented Technology Research Advancement Institution created a project to promote extensive vegetable production of processing tomatoes and onions, which can generate high crop prices even in converted paddy fields.
Unique water table control systems called the farm-oriented enhancing aquatic system (FOEAS) and underdrain flushing apparatus regulate the water table to a depth of 20 to 30 cm from the soil surface in a farm field (Kimura et al., 2018; Tsukamoto, 2015). These systems supply water through a series of pipes in dry conditions, but the pipes can also quickly drain the fields in case of excess moisture after a long period of heavy rain. Thus, they can reduce damage from an excess or lack of moisture and may lead to an improvement in yields and crop quality. One of the potential vegetable crops for using water table control systems in paddy fields is the processing tomato. At present in Hokkaido there is a total of 224,000 ha of paddy fields (MAFF, 2016), but only 2,199 ha of FOEAS (Paddy Research, 2018, private letter) and 14,464 ha in which the underdrain flushing apparatus (Hirasawa and Tsukamoto, 2016) is installed, which is about 7.4% of the total available area of converted paddy fields. The challenge is to overcome the obstacles preventing the conversion of these paddy fields to vegetable production.
Basically, there is a lack of data on the response of processing tomato plants grown in fine clay soils with excess water like that found in converted paddy fields. Some researchers have reported waterlogging or low oxygen levels in the soil when growing table tomatoes or wild varieties (Ahsan et al., 2007; Dresboll and Thorup-Kristensen, 2012; Dresboll et al., 2013; Horchani et al., 2010; Li et al., 2012; Lin et al., 2016; Morard et al., 2004), but not with tomatoes for processing or cooking. Therefore, we decided to grow processing tomatoes to understand the basic traits specific to water stress using pot experiments with ‘Natsunoshun’, a processing tomato cultivar that can grow in converted paddy fields in central Hokkaido.
This study used a two-year pot experiment to investigate the effects of excess or lack of soil moisture at various growth stages after planting on yield, growth, flowering, and fruit chemical components of ‘Natsunoshun’, a processing tomato cultivar.
This study was done in a rain-shed shelter at the Experimental Farm of the Field Science Center for the Northern Biosphere, Hokkaido University, Sapporo, Hokkaido, Japan (43° N, 141° E) in 2016 and 2017. The processing tomato cultivar ‘Natsunoshun’ (Solunum lycopercicum L.), was used as the experimental material. This particular tomato breeding line, ‘Tomato Norin-kou No. 30’, was registered as a cultivar in 2000 by the Nagano Vegetable and Ornamental Crops Experiment Station. It is now the main tomato processing cultivar in Hokkaido. It is a mid-early maturing type which does not require staking and is suitable for mechanical harvesting. The fruit quality is good for tomato juice processing and the cultivar is adapted to the cool summer climate in Hokkaido (Yanokuchi et al., 2001).
In both years, the seeding was done in 72-cell plug trays filled with potting soil (Nursery soil; Takii & Co., Ltd., Kyoto, Japan) including basal N:P2O5:K2O fertilizer at a ratio of 320, 210 and 300 mg·L−1, respectively. No additional fertilizer was supplied to the pots during the experiment. The levels of fertilizer application were approximately one-tenth of those used in conventional cultivation methods for ‘Natsunoshun’ (Chida et al., 2018). After germination, the apical meristems at the third leaf stage were pinched, creating two elongated lateral buds as the main stems of the plant, a ground creeping style of training. In early May in both years the seedlings from the cell plug trays were transplanted to 240 mm diameter poly-pots to develop the root systems. Insect and disease control were implemented according to the standard practices of the Experimental Farm of the Field Science Center for the Northern Biosphere, Hokkaido University.
Water stress treatmentsThree types of water stress treatments were created.
WET: excess soil moisture
DRY: low soil moisture
WET/DRY: WET followed by DRY
The WET treatment consisted of keeping 2–3 cm of water on the soil surface for 5–10 days. As the pots have holes in the bottom to allow for drainage, thick vinyl bags were inserted into pots to retain the water and the dissolved fertilizer (Fig. 1A, B). To create the DRY treatment, watering was discontinued for 5 days, while the plants used as controls (CONT) were given 4–7 mm·day−1 using irrigation pipes.

A young plantlet in the differentiation period of first flower truss and various fruits of the processing tomato ‘Natsunoshun’. The ‘WET’ treatment (A: photo, B: diagram) at Stage I. C: Reddish mature fruit, D: Orange and green immature fruit, E: Blossom-end rot fruit. Scale bars represent 5 cm.
The water stress treatments were conducted independently of each other at different stages of plant development: Stage I: first flower differentiation (13–27, June in 2016 and 14–28, June in 2017), Stage II: first flowering (11–20, July in both years), stage III: fruit development (10–19, August in both years). The stages I, II and III were determined by observation of the flower buds, flowers, and fruit, respectively, in 70–80% of plants. The durations of the WET treatment varied for the different stages. For stage I, it was 10 days, and stages II and III 5 days because the plants after stage II wilted completely within 10 days of the WET treatment.
MeasurementsAt harvest time, from early to mid-September in both years, yield parameters (fresh fruit yield, number of fruit per plant and average weight of fruit), and fruit harvest parameters (reddish fruit ratio, blossom-end rot fruit ratio and marketable fruit ratio) were measured. In this study, we calculated the yield parameters from the sepal-detached fruit, which included immature or blossom-end rot fruit. Each percentage value was also calculated using the all fruit number as the denominator. The evaluation of blossom-end rot and whether a fruit was marketable or not were conducted according to the standards of fruit color and size created by Numata town tomato processors in Central Hokkaido (Fig. 1C, D, E). We also calculated the crop growth rate (CGR) using all dried biomass including leaves, stems, fruit and roots, from the fruit developmental stage (growth stage III) and harvest time. At that time, the dry weight of each part of the plant was weighed after drying at 80°C for 72 h with a forced-air dryer. The plant area was calculated from the top area of the poly-pot, and was approximately 450 cm2 per plant. Next, we counted the number of flowers in late August and calculated the fruiting ratio using the number of flowers as the denominator.
The environmental parameters (soil volumetric water content, pF value, oxygen concentration in the soil, air and soil temperature, relative humidity, cumulative day radiation) were also measured. For the measurements, we used a soil moisture kit (SM150T; Delta-T Devices, Cambridge, UK), pF meter (DIK-3162; Daiki Rika Kogyo, Co., Ltd., Saitama, Japan), soil O2 sensor (MIJ-03; Environmental Measurement Japan Co., Ltd., Fukuoka, Japan), thermo recorder (TR-71U; T AND D Co., Ltd., Nagano, Japan), illuminance UV recorder (TR-74Ui; T AND D Co., Ltd., Nagano, Japan). We measured soil moisture continuously using the kit, and the O2 sensors, thermo sensors, and pF meter were imbedded in the pot soil to a depth of 15 cm. The thermo sensors to measure air temperature were set at the height of the tomato plants. The temperatures were logged automatically every hour, and the other environmental parameters were recorded between 10:00 and 12:00 am.
Tomato quality analysisFor the tomato fruit quality analysis, we sampled four fruits from a plant at harvest time, and the average value was defined as the value of a replication. Half of a fruit was squeezed to get the sap and juice which were used to measure Brix and acidity with a Brix-acid meter (PAL-BX/ACID F5 Mater Kit; ATAGO Co., Ltd., Tokyo, Japan).
The other half of the fruit was freeze-dried, powdered and stored at −80°C until the extraction of carotenoids. The extraction was done with the method developed by Takaichi and Homma et al. (Homma et al., 2012; Takaichi, 2009). Powdered samples (25 mg) were homogenized with 0.5 mL hexane, mixed for 10 min and centrifuged at 3,000 rpm for 10 min. The same process was done again, and the supernatant from each sample was corrected two times then used as the extract solution. The solution was kept at room temperature for 24 h until completely dry and re-mobilized in 100 μL of chloroform and 900 μL of acetonitrile. Then, 20 μL of the mixture was injected into a HPLC. Finally, the level of lycopene and β-carotene in the sample extract was determined by spectrophotometry at 470 nm using a UV spectrophotometer (L-7420; HITACHI, Tokyo, Japan) and 0.1 g·L−1 lycopene and β-carotene solution as an external standard. The Beer-Lambert law was used to calculate the contents.
The chromatographic separation was done with an ODS column (Inertsil ODS-P 5 μm, 4.6×150 mm; GL Sciences Inc., Tokyo, Japan) at 45°C using a pump (L-7110; HITACHI, Tokyo, Japan). The mobile phase consisted of 100% acetonitrile. The flow rate was 1.0 mL·min−1 and the spectrophotometer readings were taken at a wavelength of 470 nm. The individual carotenoids were identified using external standards using an absorption coefficient of 0.1 g·L−1 lycopene and β-carotene solution by the normalization method.
Statistical analysisThe experimental design was basically a split-split plot design with four replications (four pots per replication) according to the pot arrangement. Main plots, subplots and sub-subplots were defined as two experimental years (Y: 2016 and 2017), and three growth stages (S: I, II, and III) with four water stress treatments (T: CONT, WET, DRY, and WET/DRY). The significance in ANOVA resulted from the calculation procedures for the split-split plot design (Little and Hills, 1978). Other statistical analysis, including Tukey-Kramer’s test and Pearson’s product-moment correlation analysis were done with Statcel2 (developed by Yanai, OMS, Japan), an add-in form in Microsoft Excel 2004 for Mac.
The average maximum and minimum air temperatures in the rain-shed shelter during these experiments (from mid-June to early-September) in both years were 21.9 (±0.4)°C (max: 28.2°C, min: 12.8°C) in 2016 and 21.1 (±0.3)°C (max: 27.7°C, min: 16.2°C) in 2017, respectively (Fig. 2). The average maximum and minimum soil temperatures in the poly-pots in both years were 24.1 (±0.4)°C (max: 34.6°C, min: 16.4) in 2016 and 20.7 (±0.3)°C (max: 26.6°C, min: 16.2°C) in 2017, respectively (Fig. 2). The average temperatures of the two years were approximately the same level, but differences in temperature were found in early July (the first flowering stage) and after mid-August (the fruit development stage) for both air and soil temperatures. The average relative humidity and cumulative day radiation in the rain-shed shelter were 77.0% and 295.8 μmol·m−2·s−1 in 2016, 77.5% and 316.5 μmol·m−2·s−1 respectively.

Transitions in air and soil temperatures during the experimental period in 2016 and 2017. Air (○) and soil (●) temperatures in 2016, air (□) and soil (■) temperatures in 2017. The rectangles under Stage I, II and III represent the period of water stress treatment in 2016 (open) and 2017 (close). The values represent the daily mean temperature based on hourly measurement.
The soil volumetric water contents of each water stress treatment were 17.7–18.2% (pF: 1.9–2.0) in CONT, 37.5–42.9% (pF: 1.3–1.4) in WET and 7.1–9.1% (pF: 2.5–2.6) in DRY, respectively. In WET/DRY, the soil volumetric water content was 30.1–37.3% (pF: 1.5–1.6), and this did not decrease to the level of CONT. The oxygen concentration in the soil changed according to the soil volumetric water content: 16.3–19.6% in CONT, 2.2–5.3% in WET and 18.6–19.0% in DRY. This lower oxygenic condition is called hypoxia. The oxygen concentration in the soil in WET/DRY was 6.7–6.9%, and did not return to the level of CONT.
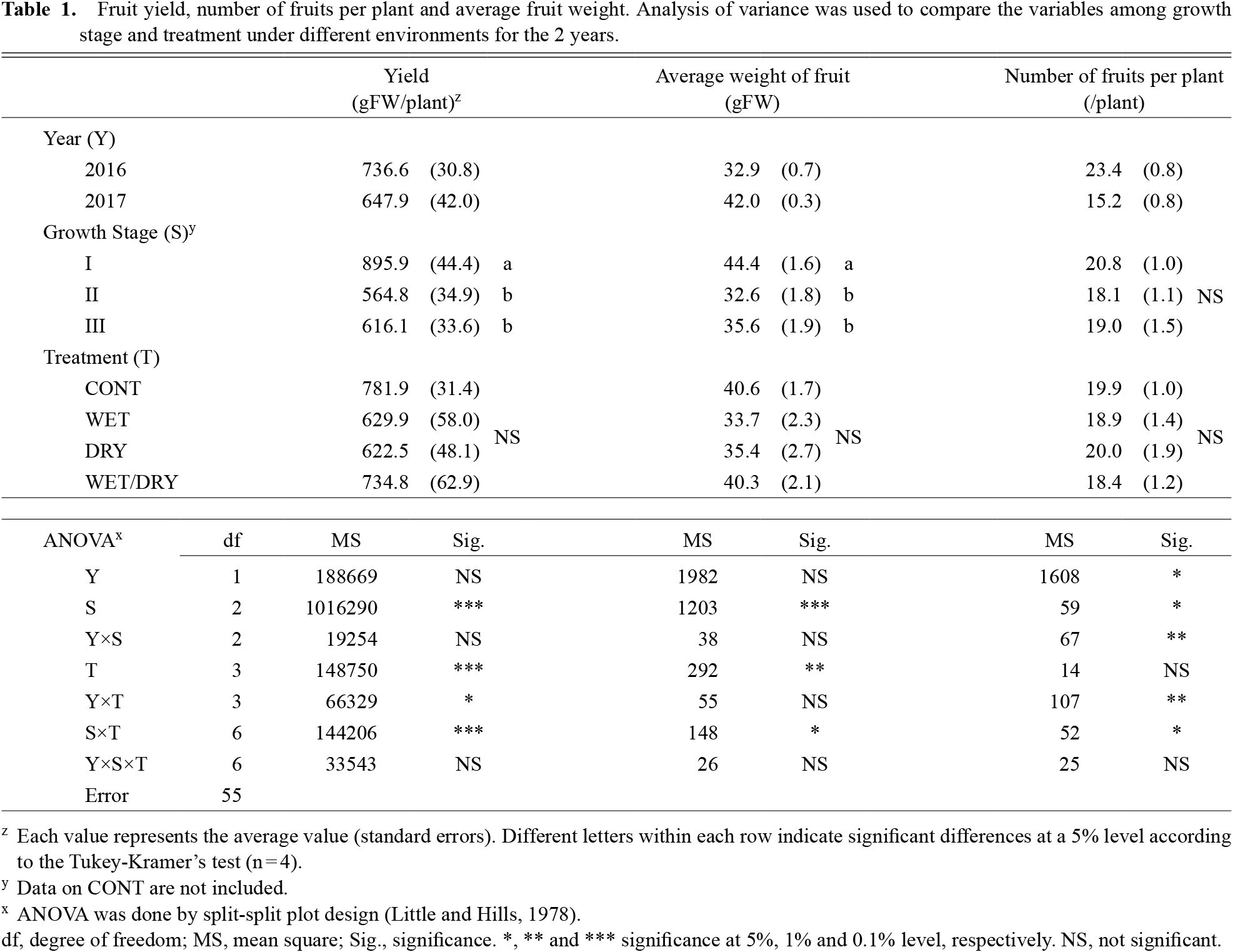
Yield and parameters relative to yieldThe difference in the yield of fresh fruit between the two years was not significant; however, the water stress after the first flowering stage reduced yields significantly (Table 1). The effect of the interaction between stage and treatment (S × T), which was significant at a 0.1% level of probability, is shown in Figure 3 using a two-year average of fruit yield per plant for each water stress treatment and in each growth stage. The yields in water stress treatments during the first flower differentiation stage were similar to the yield of CONT. However, as for the yields with water stresses, the WET treatment and DRY treatment during the first flowering stage and the WET treatment during the fruit development stage drastically reduced fruit yield during the first flower differentiation stage (Fig. 3).
Fruit yield, number of fruits per plant and average fruit weight. Analysis of variance was used to compare the variables among growth stage and treatment under different environments for the 2 years.

Differences in fruit yield in the CONT treatment and different growth stage with various water stress treatments. Data represent the means from 4 replications and bars show the standard errors. Different letters above the bars indicate significant differences among treatments and growth stages at a 5% level (Tukey-Kramer’s test).
The difference in the average weight of fruit between the two years was not significant (Table 1). The growth stage effect on the average weight of fruit was similar to that to the yield, and water stress after the first flowering significantly reduced the average weight of fruit. The differences in the average weight of fruit due to water stresses were shown to be the same as those for yield.
Fruit number per plant in 2016 was significantly higher than that in 2017 at a 5% level of probability, although it was not clear if the effect of the growth stage was similar to the other two parameters described above. The effect of the type of water stress treatment on fruit number per plant was not significant.
The relationships between fruit yield and fruit number per plant and between fruit yield and average weight of fruit are shown in Figure 4. In both relationships, positive correlations were detected. However, the average weight of fruit showed a closer relationship to yield compared with the fruit number per plant.

Relationships between fruit yield and number of fruits per plant (○) and between fruit yield and average weight of fruit (●). Circles represent each mean from 4 replications and bars show standard errors. Regression analysis shows significance at the 5% and 1% level (* and **, respectively), using Pearson’s correlation coefficient.
The effects of experimental year were not significant for the three parameters of harvested fruit: reddish mature fruit ratio, blossom-end rot fruit ratio, and marketable fruit ratio (Table 2). The reddish mature fruit ratio changed in the growth stage with the presence of water stress. The ratio was higher with stress in the fruit development stage and was lower with stress in the first flowering stage. Meanwhile, the blossom-end rot fruit ratio was higher with stresses after flowering. The marketable fruit ratio was lower with stress in the first flowering stage.

Reddish mature fruit ratio, blossom-end rot fruit ratio and marketable fruit ratio. Analysis of variance was used to compare the variables among growth stage and treatment under different environments for the 2 years.
The DRY treatment reduced the reddish mature fruit ratio to a much greater extent than the other treatments. However, for the blossom-end rot fruit ratio and marketable fruit ratio, the effects of DRY treatment were not significant. Therefore, the DRY treatment put fruit maturity at risk, although the fruit number per plant with the DRY treatment was almost equal to that of CONT (Table 1). Fruit number per plant in the DRY treatment was determined by the fruiting ratio rather than the number of flowers per plant (Fig. 5). There was a positive correlation between fruit number per plant and the fruiting ratio, and the DRY treatment during the fruit development stage increased both of these. No relationship was detected between fruit number per plant and the fruiting ratio or between the fruit number per plant and the number of flowers per plant in the other treatments.

Relationships between number of fruits per plant and number of flowers (A) and fruiting ratio (B). Circles represent each mean from 4 replications and bars show standard errors. Regression analysis shows significance at the 1% level (**), using Pearson’s correlation coefficient. The other formulas are not significant (NS).
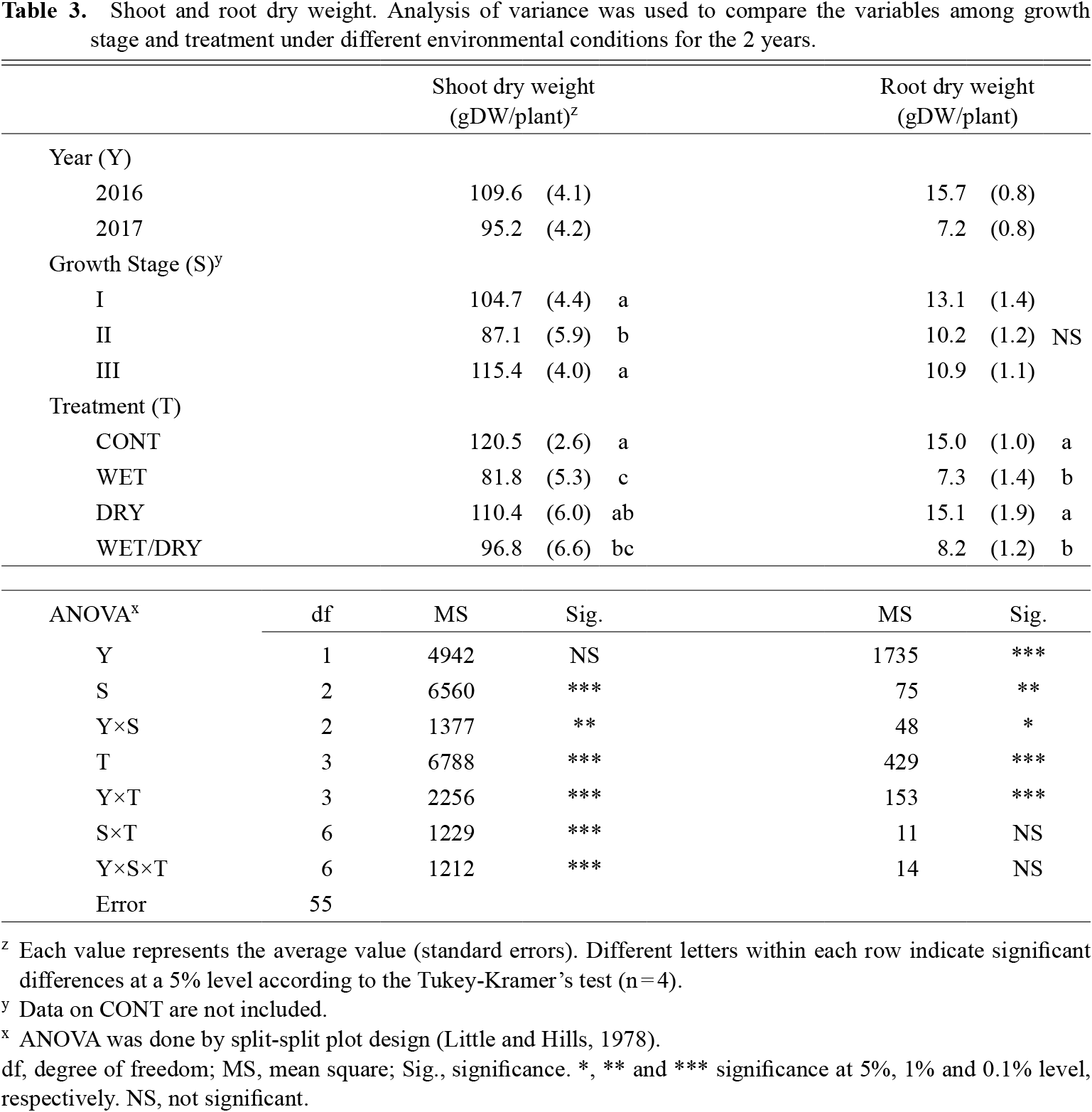
The effects of experimental year were not significant regarding shoot dry weight (Table 3). However, the effect of the growth stage with water stress and the type of water stress were significant. Water stress during the first flowering stage, especially the WET or WET/DRY treatments, drastically reduced shoot dry weight.
Shoot and root dry weight. Analysis of variance was used to compare the variables among growth stage and treatment under different environmental conditions for the 2 years.
The root dry weight in 2016 was significantly greater than in 2017. The differences in the root dry weight caused by treatments are shown in Table 3. The effect of excess soil moisture was quite significant, and WET or WET/DRY treatments reduced root dry weight by half.
The crop growth rate (CGR) between the fruit developing stage and harvest time was significantly higher in the CONT treatment than the WET or WET/DRY treatments during the first flowering stage, or the WET treatment during the first flower differentiation stage (Fig. 6).

Differences in crop growth rate (CGR) in the CONT treatment and different growth stages with various water stress treatments. Data represent means from 4 replications, and bars show standard errors. Different letters above the bars indicate significant differences among treatments and growth stages at a 5% level (Tukey-Kramer’s test).
Chemical components of fruit, the Brix, acidity and water content were measured (Table 4). The Brix of fruit in 2016 was significantly higher than in 2017 and the Brix increased with water stress after the first flowering stage, especially with the DRY treatment. Similar to Brix, the differences detected in fruit acidity with water stresses at the different growth stages, especially the DRY treatment, increased fruit acidity in the CONT treatment. The water content of fruit decreased significantly with water stress during the first flowering stage, which affects Brix and acidity.

Brix, acidity, brix-acid ratio and water content. Analysis of variance was used to compare the variables among growth stage and treatment under different environments for the 2 years.
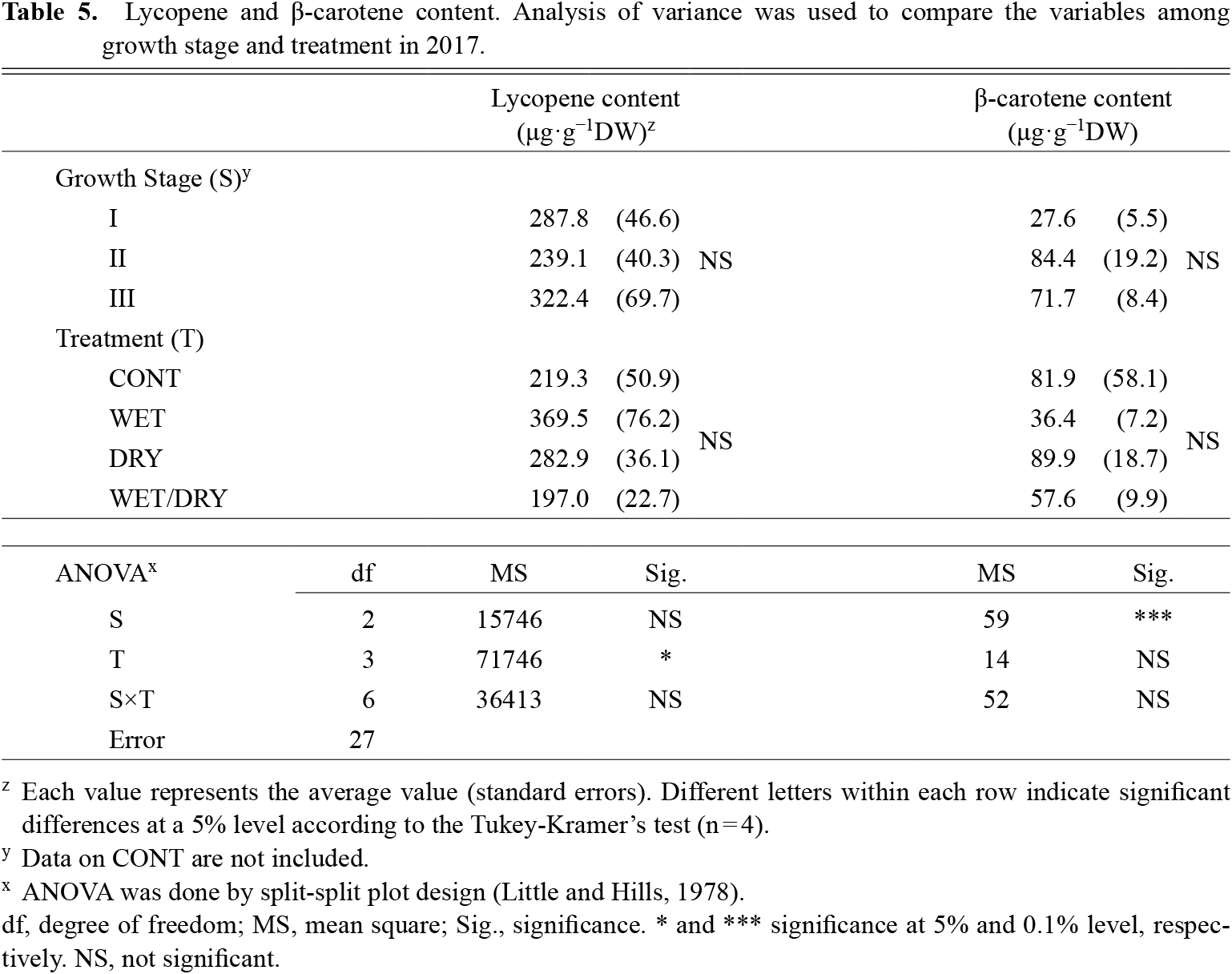
Other fruit chemical components, lycopene and β-carotene, were also measured (Table 5). The carotenoids were analyzed in 2017. For fruit lycopene content, the effect of growth stage with water stress was not significant; however, the treatment effect was significant. In contrast, for fruit β-carotene content, the effect of the different water stressed were not significant, while that of growth stage was significant.
Lycopene and β-carotene content. Analysis of variance was used to compare the variables among growth stage and treatment in 2017.
In this study, yields were lower than those of field production. The yield of marketable ‘Natsunoshun’ fruit was 2.5 kg/plant (Chida et al., 2018); however, for the CONT in this study it was approximately 0.8 kg/plant in 2016 (Table 1). Furthermore, the marketable yield decreased to 0.27 kg/plant in 2017, one-third of the 0.8 kg/plant level in 2016 (Table 2). One of the reasons for the lower yield level may be root volume restriction in the poly-pots (Figure 1A). For the processing tomato, root volume restriction reduced shoot biomass and fruit numbers (Saito et al., 2008). However, we believe that the low yields in the pot experiment can be attributed to lower fertilizer levels, which were approximately one-tenth those of the conventional cultivation methods used with ‘Natsunoshun’ (Chida et al., 2018). We conducted this study as a simple model experiment with the assumption that tomato plantlets in pots would respond to extreme water stresses like plantlets in a cultivated field.
Meanwhile, the deleterious effects of waterlogging have been partially offset by a top dressing of nitrogen in cotton (Hodgson and MacLeod, 1988), soybean (Haq and Mallarino, 2000) and wheat (Tani et al., 2015). In the current pot experiment, it seems quite probable that the low fertilizer level increased the waterlogging injury after the first flowering stage. However, no difference in the fruit yield from the wet condition was detected between the first flowering stage and the fruit development stage (Fig. 3). Furthermore, the CGR of tomato plants under WET treatment at the flowering stage recovered to the level of CONT at the fruit development stage (Fig. 6). These results suggested that the first flowering stage of the ‘Natsunoshun’ tomato was the most sensitive to the excess soil moisture regardless of the nutritional status. Even so, we need to do further experiments under various fertilizer conditions to clarify the effect of nutrient deficiency on waterlogging injury.
In general, yield and quality of vegetables, as well as processing tomatoes (Giuliani et al., 2016), are affected by fluctuations in air temperature during cultivation (Bisbis et al., 2018). In this study, the differences in temperature were large, but yields in both years were not significantly different (Table 1). This shows that the effects of environmental changes were smaller than the effects from water stress and type of water stress at the growth stages in this study.
These results show how yield is impacted by water stress during the growth stage after the first flowering, and particularly how WET and DRY treatments caused a drastic reduction in yields (Table 1). In contrast, the WET/DRY treatment did not reduce yields. We concluded that the DRY treatment offset the negative effect of the earlier WET treatment. Lower soil moisture after waterlogging accelerates drought injury in soybeans, because of shallow root depth (Shimane Prefectural Government, 2018, http://www.pref.shimane.lg.jp/industry/norin/gijutsu/nougyo_tech/kenyui/sisin_manyuaru/ine-sisin/ine-sisin.data/27.820.pdf), but in this study using processing tomatoes, the DRY treatment after the WET treatment may have minimized waterlogging injury, thus minimizing the normal reduction in yields. In soybeans, waterlogging allows the shallow root system to be easily impacted by drought stress. In this pot experiment, the processing tomato’s roots developed as whole in the pot, which means there were no differences in root density at any depth in the pot. This resulted in the changes in water status in the process of transitioning between the DRY and WET treatments being moderate with less severe stress.
Some improvements in the fruit yields were seen in the water stress treatments at the first flower differentiation stage compared with that of CONT (Fig. 3). The results indicate that the tomato plant is tolerant of water stress if it occurs in a growth stage before flowering. Specifically, the WET treatment, or even a longer duration of waterlogging before flowering, can possibly increase yield. A shortage of water for an appropriate duration during a seedling’s nursery stage in cucurbitaceous crops (Mundalia et al., 2015) and other vegetables (Oda, 2007) can positively affect plant vigor. According to the results from this study, using subirrigation with optimal timing may increase a processing tomato’s productivity, even if there is excess soil moisture.
There was however, a tendency for reduced yields if the WET treatment came after the first flowering stage, even for a short period of waterlogging (Fig. 3). Possibly sensitivity to hypoxia has a more immediate effect. Li et al. (2015) reported that aeration of soils during the flowering stage or the fruit developing stages can increase tomato yield and fruit size. This agrees with the results from this study which showed that the WET treatment reduced yield by causing hypoxia after the first flowering stage.
The fluctuations in the average weight of fruit were similar to those of yield (Table 1). They were not affected by the year the effect and decreased with water stress after the first flowering stage, especially with WET or DRY treatments. This synchronism between yield and average fruit weight can be seen by the high correlation coefficient (Fig. 4). In other words, water stress has a relatively strong effect on the average fruit weight, and influences yield via a decrease in fruit size. In a previous study, Li et al. (2015) reported that soil aeration had a positive effect on increasing fruit size. In this study, we also considered that hypoxia in the rhizosphere caused by waterlogging may have limited fruit size, resulting in smaller yields of processing tomatoes.
The fruit number per plant also showed a positive correlation to yield (Fig. 4), meaning a large fruit number results in a higher yield. Fruit number per plant was affected by a year effect and in 2016 it was higher than in 2017 (Table 1). The differences in annual values may have been caused by higher air temperatures during the first flowering stage in 2017 than in 2016, and in 2016 during the fruit development stage they were higher than in 2017.
Interestingly, the fruit number per plant also demonstrated a close positive correlation to the fruiting ratio only in the DRY treatment plants (Fig. 5). These results suggest that water stress with the DRY treatment in 2016 was more severe than in 2017 during the fruit developing stage and that this promoted a wide range in the fruiting ratio which in turn generated a wide range in the fruit number. As shown in Figure 5B, the fruit number values and fruiting ratio were higher in the fruit developing stage, the first flowering stage and the first flower differentiation stage, respectively. The mechanism that causes the decrease in the values is not clear, but it suggests that a higher fruiting ratio results in higher fruit numbers with the DRY water stress treatment. In contrast, flower number had no relationships with any water stress treatments (Fig. 5A). A lack of water can delay and reduce flowering of tomato plants (Saito, 1969), but in this study we could not establish a relationship.
One more characteristic of the DRY treatment was a decrease in a fruit harvest parameter, the reddish mature fruit ratio (Table 2). There was a significant decrease in the number of reddish mature fruit, resulting in a marketable fruit ratio at a 5% level of significance for the treatment effect. The main growth stage that has the greatest impact on fruit harvest parameters is the first flowering stage, and lack of moisture may delay maturation and increase morbidity of blossom-end rot.
The causal association between dry conditions and blossom-end rot was established by Yoshida et al.(1997) and the reasons cited were a shortage of calcium fertilizer (Manishi et al., 1996) and the slackening of calcium transportation in the tomato plant body (Ikeda et al., 2017). The direct cause of blossom-end rot is considered to be the demand for calcium in the fruit surpassing the supply, leading to a calcium deficiency in the fruit (Ooyama et al., 2016). The results of this study suggest that the WET or DRY treatments cause a failure of root water uptake, eventually reducing the uptake of calcium as well, and thus lowering the calcium concentration of the fruit. The solution to prevent the failure is to inhibit transpiration by defoliation treatment (Sato et al., 2004). Ethylene treatment that induced leaf fall contributed to defoliation and reduced immature fruit, but it could still stimulate blossom-end rot (Saure, 2001). The Blossom-end rot in this study may have been controlled effectively by applying calcium to the fruit (Yoshida et al., 2014).
In this study, many leaves were completely wilted after the DRY treatment, which delayed maturation, and resulted in a decrease in the fruit harvest parameter. For processing tomatoes, harvesting them all at once is convenient as mechanical harvesting is used to save labor. Therefore, synchronized fruit maturation is a desirable characteristic for processing tomato production. The results suggest that the DRY treatment may not be suitable for one-step mechanical harvesting. Generally, water stress induces crop maturation, although the DRY treatment in this study delayed fruit maturation (Table 2). In a previous study using tomatoes, temporal heat stress (Iwahashi et al., 1999) and leaf damage (Calvo et al., 2007) during the growth stage induced delayed fruit maturation. Transpiration may be inhibited temporarily during non-irrigation, and elevate plant temperature and leaf wilting.
As well as the DRY treatment, the WET treatment, especially after flowering, also caused a reduction in tomato yield (Fig. 3). Waterlogging severely limited tomato plant biomass, especially in the roots, which was half that of the CONT and DRY treatment plants (Table 3). The absorption of water and nutrients places a demand on the oxygen concentration in the rhizosphere (Morard and Silvestre, 1996). Actually, nitrogen uptake by whole roots in 5-week old tomato seedlings rapidly decreased with five days of waterlogging (Dresboll and Thorup-Kristensen, 2012). Previous studies of pot experiments using 2 to 5-week old tomato seedlings showed that the root elongation rate drastically slowed immediately after a waterlogging treatment (Dresboll et al., 2013) and apoptosis occurred five days later (Ahsan et al., 2007). Thus, root reduction and the loss of nutrients for photosynthesis slows the plant growth rate and this slower growth rate reduces the biomass required for photosynthesis, which in turn slows the growth rate further. ‘Natsunoshun’, the experimental material for this study was rated relatively sensitive to excess soil moisture in the author’s preliminary test of processing tomato cultivars that have been released in Japan (data not shown). Therefore, if the ‘Natsunoshun’ cultivar is to be cultivated in converted paddy fields, we suggest avoiding wet conditions after the first flowering stage or to breed cultivars that incorporate traits of morphologically stable roots capable of acclimating to fluctuations in rhizospheric oxygen concentrations.
Generally, soil moisture affects the chemical compositions of fruit, and the concentrations were high under appropriately limited irrigation (Asano, 1993). The DRY treatment in this study reduced the fruit harvest parameter; however both Brix and acidity increased with the DRY treatment as seen in a previous study (Table 4). Although the fruit water content also decreased with water stress in comparison to the CONT fruit, the level of change was insignificant (up to 0.2%) and not enough to change the concentrations.
Basically, processing tomatoes have 2–3 times more lycopene than fresh market tomatoes (Sargent and Maynard, 2012) and the lycopene’s bioavailability is high after heat-cooking (Gärtner et al., 1997). A difference in fruit lycopene content was seen in the water stress treatments in this study, including a slight increase with the DRY treatment (Table 5). Limiting water during cultivation of tomatoes can increase carotenoids, lycopene, and β-carotene in fruit (Favati et al., 2009). On the other hand, the WET treatment may contribute to the accumulation of fruit lycopene, but not β-carotene, unlike the DRY treatment. In a previous study using fresh market tomatoes, waterlogging during the fruit development stage reduced carotenoids (Horchani et al., 2010). Compared to the WET treatment, the DRY treatment could be used to improve taste and nutrient contents if the problems of maturation and blossom-end rot that accompany lack of moisture can be avoided.
Water stress during the growth stage, after the differentiation of the first flower (stage I), improved yield compared with the other two stages (Table 1), but the Brix of the fruit was reduced (Table 4). Sugar concentrations of tomato fruit can generally be increased by applying water or salt stress to plant root zones before harvest (Johkan et al., 2014; Saito et al., 2006). This study suggests that the WET treatment induced plant wilting, but not a high concentration of carbohydrates in the fruit. The Brix of fruit with the DRY treatment was higher than that with the DRY treatment after the WET treatment (Table 4).
The balance between carbohydrates and acidity in tomato fruit is important for a better taste. An indicator of the balance is a sugar-acid ratio greater than 12.0 (DAES, 1987). The highest sugar-acid ratio was 8.0 in the CONT, lower than the12.0 required for good tasting fruit (Table 4). Neither of the water treatments in this study improved the quality of fruit taste.
In conclusion, we identified the growth stages in a processing tomato in which water stress, less or excess soil moisture, have the greatest effects. The mechanisms of abiotic injuries in both situations can be explained by two different processes: biomass damage from excess water, and quality and fruiting ratio damage due to lack of moisture, respectively. Considering the production of processing tomatoes in converted paddy fields in Hokkaido in the future, we believe it is important to avoid excess moisture after the first flowering stage and if the issues of increased immature fruit and blossom-end rot fruit are solved by limiting soil moisture after flowering, it will be possible to harvest good tasting and highly nutritious fruit.
We would like to extend our appreciation to Mr. M. Torikoshi of the Hokkaido Research Organization, Agricultural Research Development, Ornamental Plants and Vegetables Research Center, and Dr. R. Nakamura and Mr. Y. Oohashi of the Hokkaido Research Organization, Agricultural Research Development, Central Agricultural Experimental Station for providing helpful suggestions. We would like to thank Ms. T. Chida of Sorachi Agricultural Extension Center and Mr. H. Sakamoto of Breeding Section, Nagano Vegetable and Ornamental Crops Agricultural Experiment Station for preparing the experimental materials. We would also like to thank Mr. T. Tsunoda, Mr. S. Ichikawa and Mr. H. Nakano of the Field Science Center for the Northern Biosphere, Hokkaido University for assisting with the preparation of the experimental system and field.